Chemical Equilibrium - Chp 17
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
19 Terms
Reversible Reaction
is one in which the products react to give back the reactants, the reaction is going in both directions
Chemical Equilibrium
a state of dynamic balance in a reversible reaction where the rate of the forward reaction is the same as the rate of the backward reaction
Dynamic state
a state in which the reactants are continuously forming products and the products are continuously forming reactants
Dynamic Equilibrium
the rate of forward reaction is equal to the rate of the backward reaction
Le Chatelier’s Principle
states that is a stress is applied to a system at equilibrium, the system re-adjusts to relieve the stress applied
Name 2 industrial applications of Le Chatelier’s Principle
Manufacture of Ammonia by the Haber Process
Manufacture of Sulfuric Acid by the Contact Process
Write an equation for the manufacture of ammonia + state whether exothermic or endothermic going forward:

What does Le Chatelier’s Princple suggest for optimal ammonia production + what is the problem + what is the compromise?

Temperature 500 degrees C
Pressure of 200 atmospheres
Write an equation for the manufacture of sulfuric acid + state whether exothermic or endothermic going forward:
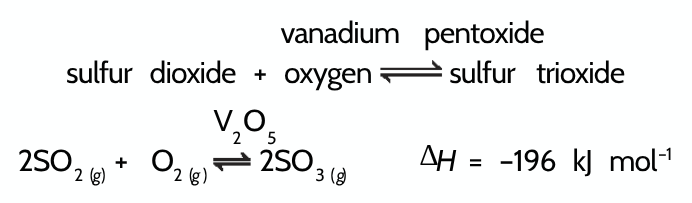
Exothermic
What does Le Chatelier’s Princple suggest for optimal sulfuric acid production + what is the problem + what is the compromise?
High Pressure - shifts equilibrium to the product side/right
Low temperature - shifts equilibrium to the product side/right
Problem
High pressure - explosion more likely + more expensive
Low temperature - rate of reaction too slow
Compromise
Pressure just below atmospheric pressure
Temperature of 450 degrees C
How do you write an expression for Kc?
[product]no. moles [product]no. moles / [reactant]no. moles [reactant]no. moles
square brackets represent concentration in moles per litre
What does Kc express?
the larger the value of Kc the more the equilibrium is shifted to the product side
What effects the value of Kc?
Kc is always constant for the given chemical equation and temperature
changes in temperature alone affect Kc
How do you find the value of Kc?
find the expression for Kc
find the concentration of reactants and products in moles per litre
sub into the Kc expression
How do you find the concentration of the products when given the value of Kc and the initial concentration of the reactants?
find the expression of Kc
sub known values into table
find the conc at equilibrium of the reactants and products in terms of x
sub values into Kc expression and form a quadratic equation
find x, choosing the most logical value between the two
sub values in to find the concentrations at equilibrium
Write an equation for the experiment to illustrate Le Chatelier’s principle
Iron (III) Chloride + Potassium Thiocynate ⇌ Ferrocyothyate + Chloride ions
Fe3+Cl3- + CNS- ⇌ Fe(CNS)2+ + 3Cl-
yellow colourless red
What 2 variable are investigated in the experiment?
concentration
temperature
Describe the steps in the investigation of the effect of concentration on the position of equilibrium
some Iron (III) Chloride solution and one drop of Potassium Thiocynate solution are mixed in a test tube
red colour is observed, due to the formation of Ferrocyothyate
the equilibrium has shifted to the right side of the equation
some dilute HCl acid is added until red disappears
the equlibrium shifts left to use up added Cl- ions
some Potassium Thiocynate is added, and a red colour is observed
the equilibrium has shifted to the right side of the equation to use up substance added
Describe the steps in the investigation of the effect of temperature on the position of equilibrium