chem exam 2 - unit 4 electron configuration
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
24 Terms
electron configuration
the arrangement of electrons in the energy shells (energy levels)
electrons exist in layers called __ (energy levels)
shells
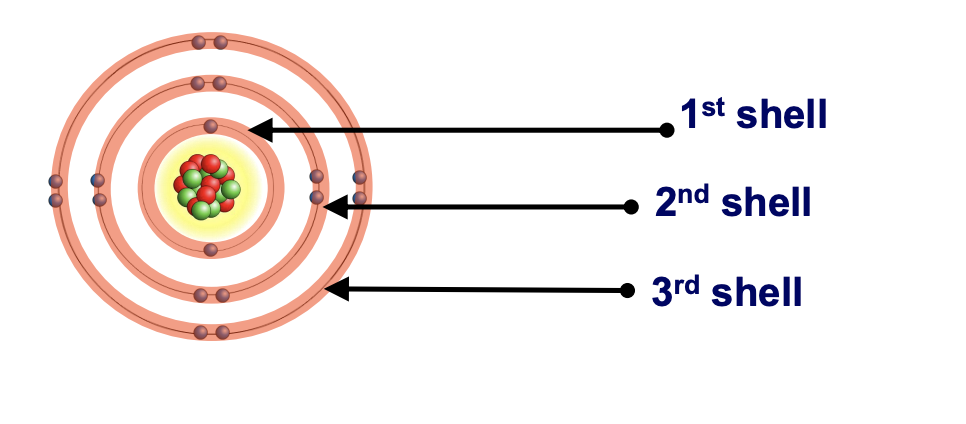
electrons will fill the shells nearest the __ first
1st (K) shell holds a maximum of __ electrons
2nd (L) shell holds a maximum of __ electrons
3rd (M) shell holds a maximum of __ electrons
equation to solve maximum # of electrons per shell: 2n²
n = number of shell layer
nucleus
2
8
18
K SHELL:
subshell name: 1s
maximum # of electrons present in shell: 2
distribute the electron in the subshell: 1s²
L SHELL:
subshell name: 2s, 2p
maximum # of electrons present in shell: 8
distribute the electron in the subshell: 2s² and 2p^6
M SHELL:
subshell name: 3s, 3p, 3d
maximum # of electrons present in shell: 18
distribute the electron in the subshell: 1s²
N SHELL:
subshell name: 4s, 4p, 4d, 4f
maximum # of electrons present in shell: 32
distribute the electron in the subshell: 4s², 4p^6, 4d^10, 4f^14
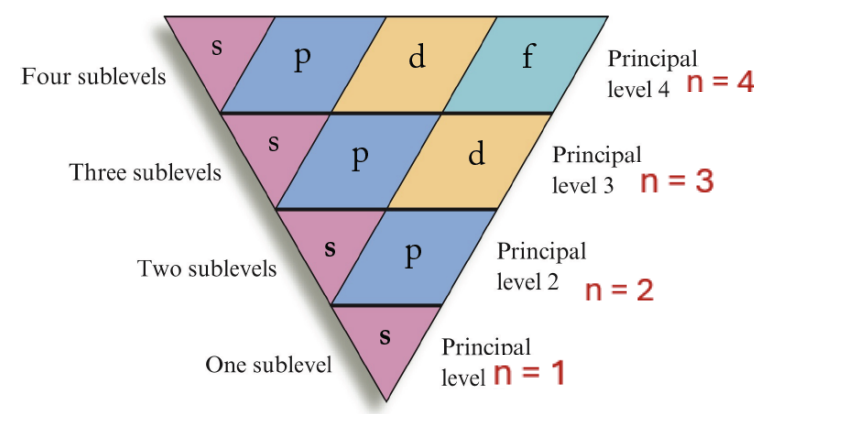
shell: n = 4 , # of subshells = 4 , letters specifying subshells = s p d f
shell: n = 3 , # of subshells = 3 , letters specifying subshells = s p d
shell: n = 2 , # of subshells = 2, letters specifying subshells = s p
shell: n = 1 , # of subshells = 1, letters specifying subshells =
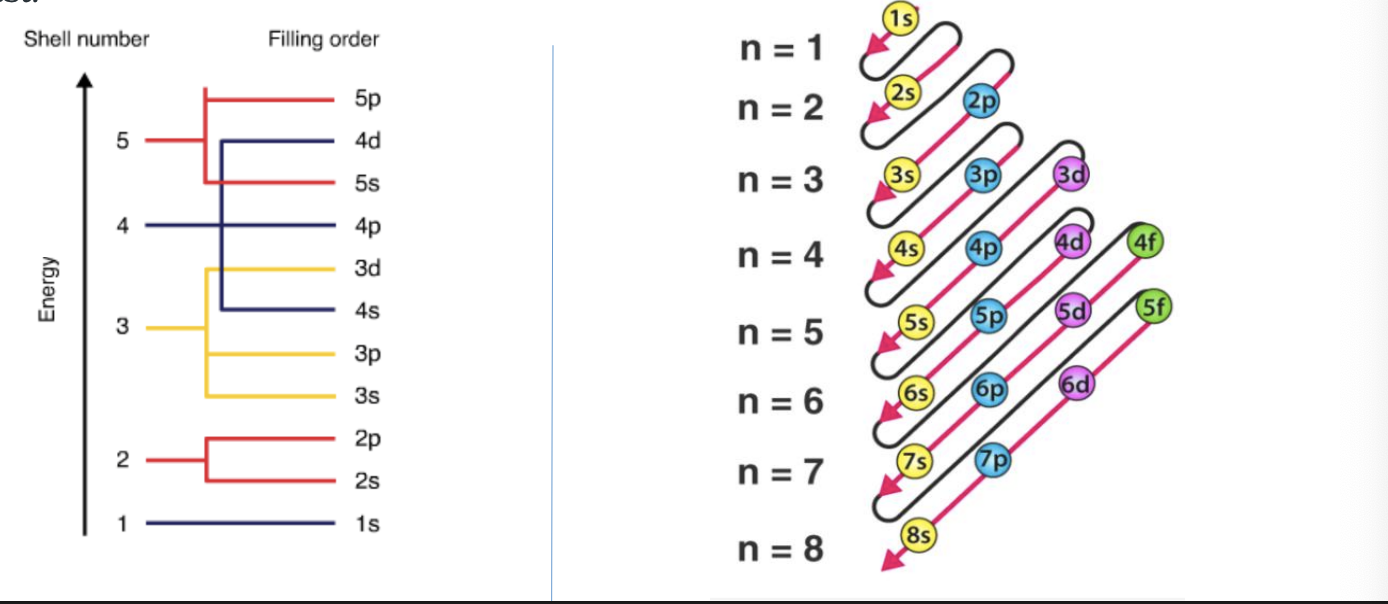
Aufbau principle
electrons in different orbitals are filled in the increasing order of their energy
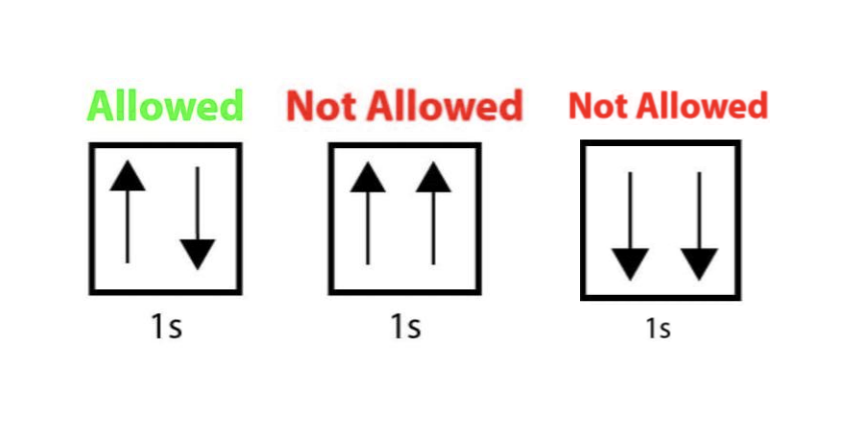
Pauli Exclusion Principle
no more than two electrons can occupy the same orbital
two electrons in the same orbital must have opposite spins
Hund’s Rule
every orbital in a sublevel is singly occupied before any orbital is doubly occupied
all of the electrons in singly occupied orbitals have the same spin
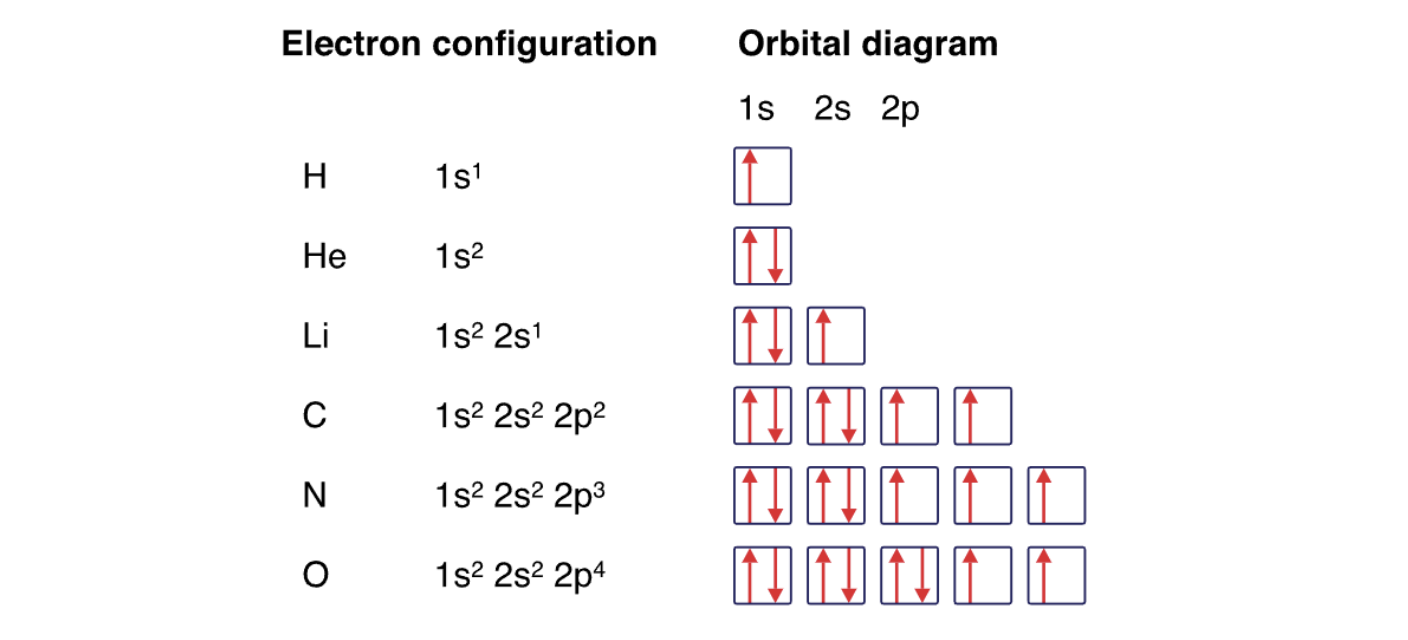

steps for writing electron configurations
know the number of electrons: it is equal to the atomic number of the element (unless it is an ion)
follow the order of orbitals: use Aufbau diagram
ex: Potassium (K) – Atomic Number 19
19 electrons
Configuration:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹
ex: Bromine (Br) – Atomic Number 35
35 electrons
Configuration:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁵

steps for electron configuration for cations (positive ion)
Find the electron configuration of Mg²+
Step 1: Find the electron configuration of neutral Mg atom.
Mg (12) = 1s² 2s² 2p^6 3s²
Step 2: If the ion is ‘cation (positive ion)’, then SUBTRACT the same number of electrons from the outermost shell (valence shell)
in this case, +2 is the charge
remove two electrons from outermost shell (Valence shell) → in this case it is 3s²
electron configuration of Mg2+ is now 1s² 2s² 2p^6
steps for electron configuration for anions (negative ion)
Find the electron configuration of P³-
Step 1: Find the electron configuration of neutral Phosphorous (P) atom
P (15) = 1s² 2s² 2p^6 3s² 3p³
Step 2: If the ion is ‘anion (negative ion)’, then ADD the same number of electrons to the outermost shell (valence shell)
in this case, -3 is the charge
add three electrons to the outermost shell (valence shell) → in this case it is 3p³
electron configuration of P³- is now 1s² 2s² 2p^6 3s² 3p^6
identifying the neutral element for electron configurations
add up all the numbers in the superscripts
match the sum value to an element with the same atomic number
ex: 1s²2s²
2+2 = 4
element with atomic number of 4 = Beryllium
identifying the charged element for electron configurations
same exact counting steps as for a neutral element
after that, ADD the ion charge if +, or SUBTRACT the ion charge if -, to the sum value
match the new value to the atomic number of element with the ion charge amount with it
ex: Which ion with a +1 charge has the following electron configuration?
1s² 2s² 2p^6 3s² 3p^6 3d^10 4s² 4p^6
= Rb+1
ex: Which ion with a -2 charge has the following electron configuration?
1s² 2s² 2p^6 3s² 3p^6 3d^10 4s² 4p^6
= Se-2
Bohr’s model:
moving up, electrons __ energy
moving down, electrons __ light energy
absorbs
emits
Principal quantum number (n)
known as n
describes the main energy level occupied by the electron
as n increases, the ‘size’ of atom increases too
the bigger the value of n = the higher the energy
n must be a positive integer value starting from 1 (n = 1,2,3 etc.)
n will never be a negative integer or zero
Azimuthal quantum number (l)
angular momentum:
specifies the shape of an orbital aka subshell
for every principle shell (n), there are 1+ subshell
4 diff subshells:
s - “sharp”
p - “principle”
d - “diffuse”
f - “fundamental”
magnetic quantum numbers (ml)
determines the orientation of the orbital
represented by symbol: ml
spin quantum number (ms)
symbol: ms
does not depend on another quantum number
designates direction of the electron spin
either a spin of +1/2 (shown by ↑) or -1/2 (shown by ↓)
positive ms = electron of an upward spin (aka “spin up”)
negative ms = electron of a downward spin (aka “spin down”)
valence electrons
are the outermost shell electrons
inner shell electrons = core electrons
only VE can chemical bond
identifying valence electrons
the number equal to the last digit of the group in P table = their valence electrons
slide 42: how do you identify the valence electrons and core electrons of an element from the electron configuration?
ionization energy
energy needed to remove an electron from an atom
ionization energy increases when the atomic radius decreases
how to find the ionization energy of elements
use the periodic trends:
increases across a period (→ left to right) bc of more protons = more attraction = harder to remove electrons
decreases down a group (↓ top to bottom) more electron shells = electrons farther from nucleus = easier to remove
ex: Who has higher IE: Mg or Na?
Mg is to the right of Na → Mg has higher IE.
ex: Which has the lowest IE: Li, Na, or K?
K is furthest down → K has the lowest IE
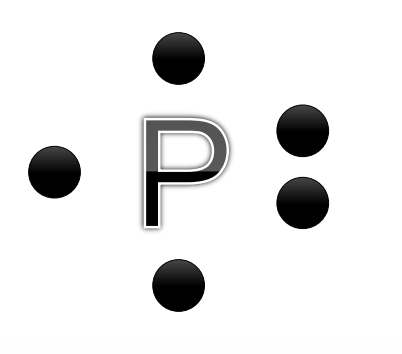
how to draw a lewis dot structure
represents the valence shell electrons
steps:
find element on P table
determine # of valence electrons by looking at the group
it starts at the right moving clockwise around symbol
ex: P (group 5)
slide 53: octet rule
tendency of atoms to prefer to have eight electrons in the valence shell
atoms with less than 8 electrons in their valence shell either lose or gain electrons to form stable compounds
only s and p orbitals are involved in octet rule
ex: Electron Configuration of Neon (Ne) 1s² 2s² 2p^6 octet state
ex: Electron Configuration of Argon (Ar) 1s² 2s² 2p^6 3s² 3p^6 octet state