Biochem 501 - Unit 3 Metabolic Pathways
1/96
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
97 Terms
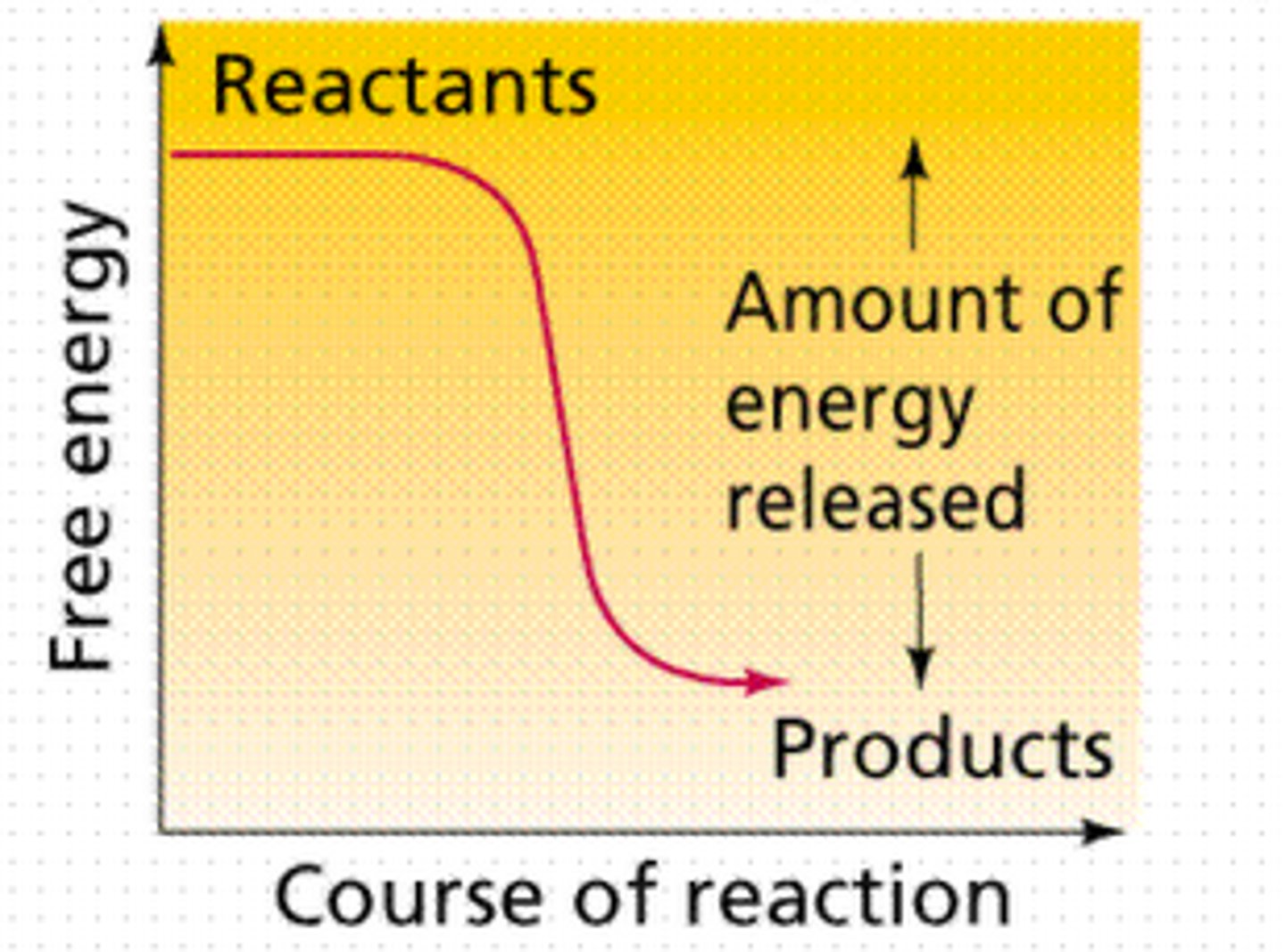
exergonic
reactants to products
releases energy
-G
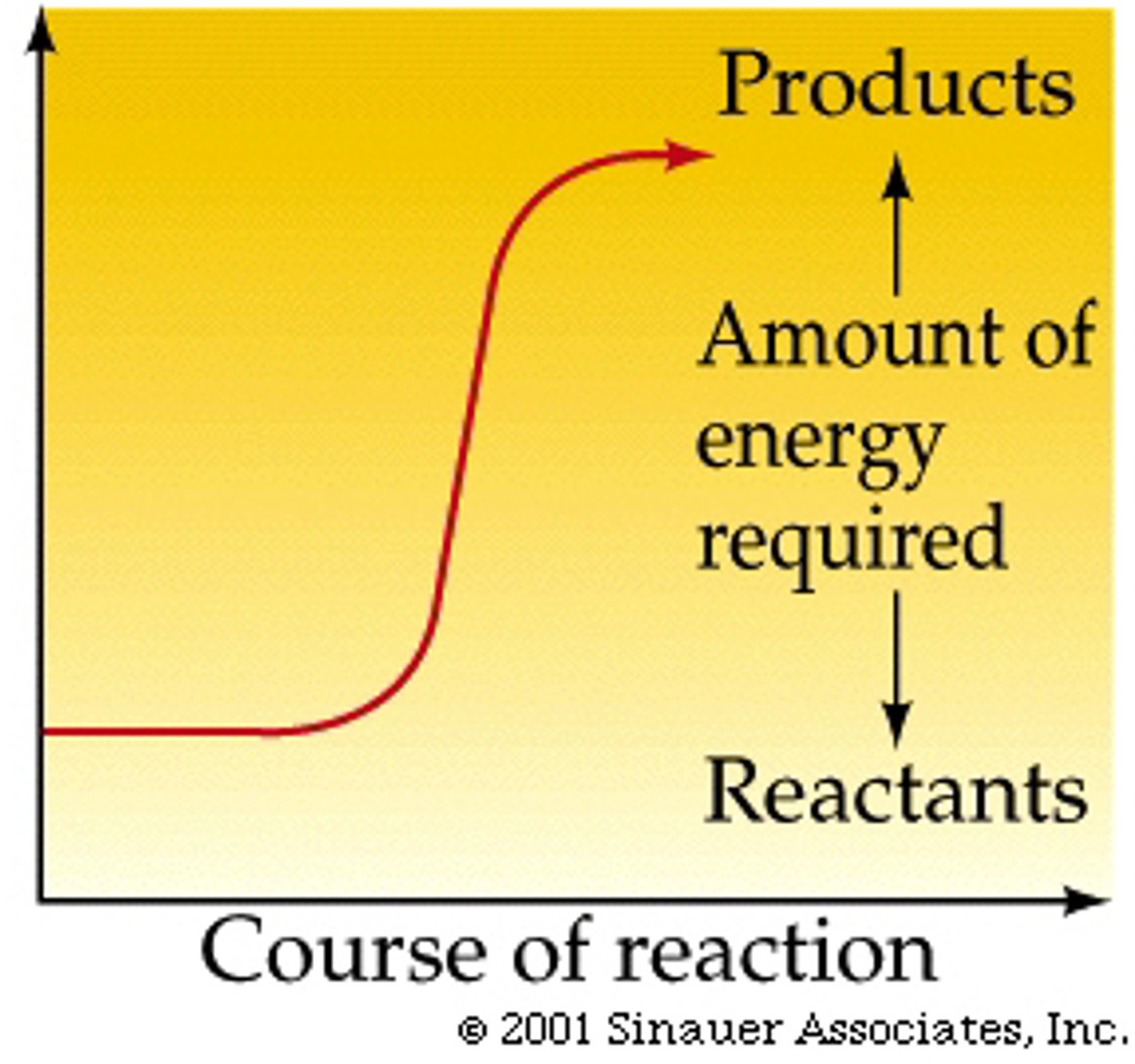
endergonic
products to reactants
input of energy
+G
exothermic
bonds formed and heat released
endothermic
bonds broken and heat absorbed
net reactions
go towards equilibrium and energy is available as equilibrium is approached
prepatory phase of glycolysis
phosphorylation of glucose and its conversion to glyceraldehyde 3-phosphate
-endergonic
-Uses (-2ATP)
payoff phase of glycolysis
Oxidative conversion of glyceraldehyde 3-phosphate to pyruvate and the coupled formation of ATP and NADH
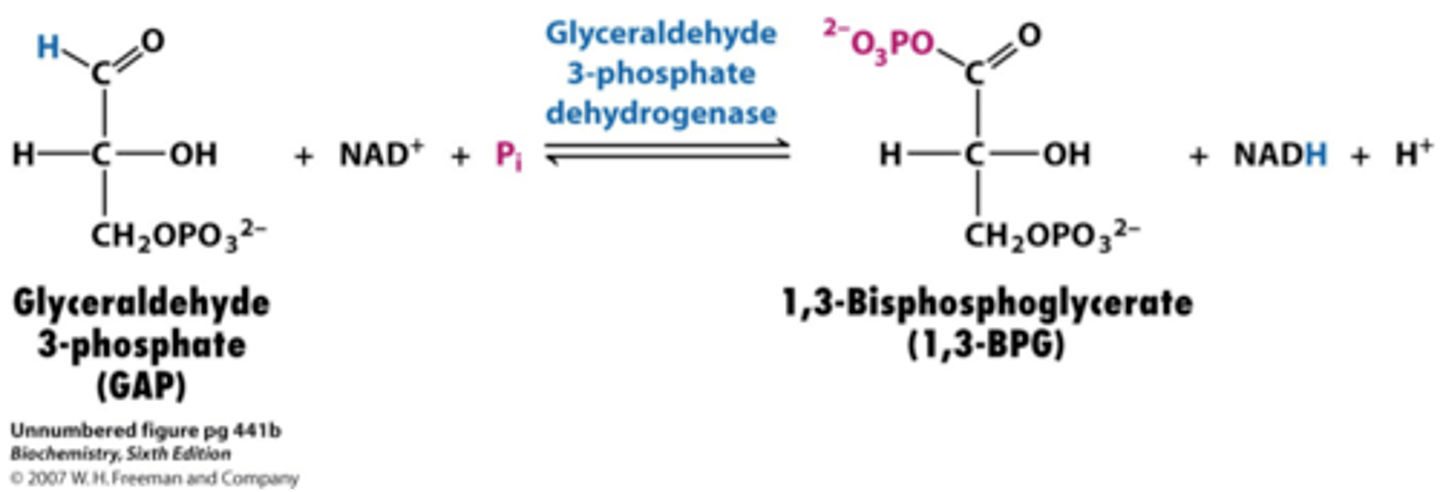
dehydrogenase reaction glycolysis
produces 2NADH by oxidation, only redox reaction in glycolysis
step6
products of glycolysis
2 ATP, 2 NADH, 2 pyruvate
reactants of glycolysis
glucose, 2 ATP, 2 NAD+
NADH formation
NAD+ must be oxidized and is a transfer of 2e- and 2H+
ATP as a substrate and inhibitor
binds to both the active site of the enzyme and to the separate allosteric site.
enzymes catalyze irreversible steps
this avoids futile cylcing
fermentation
ways to regenerate NAD+ from NADH w/out O2 to maintain glycolysis to produce ATP
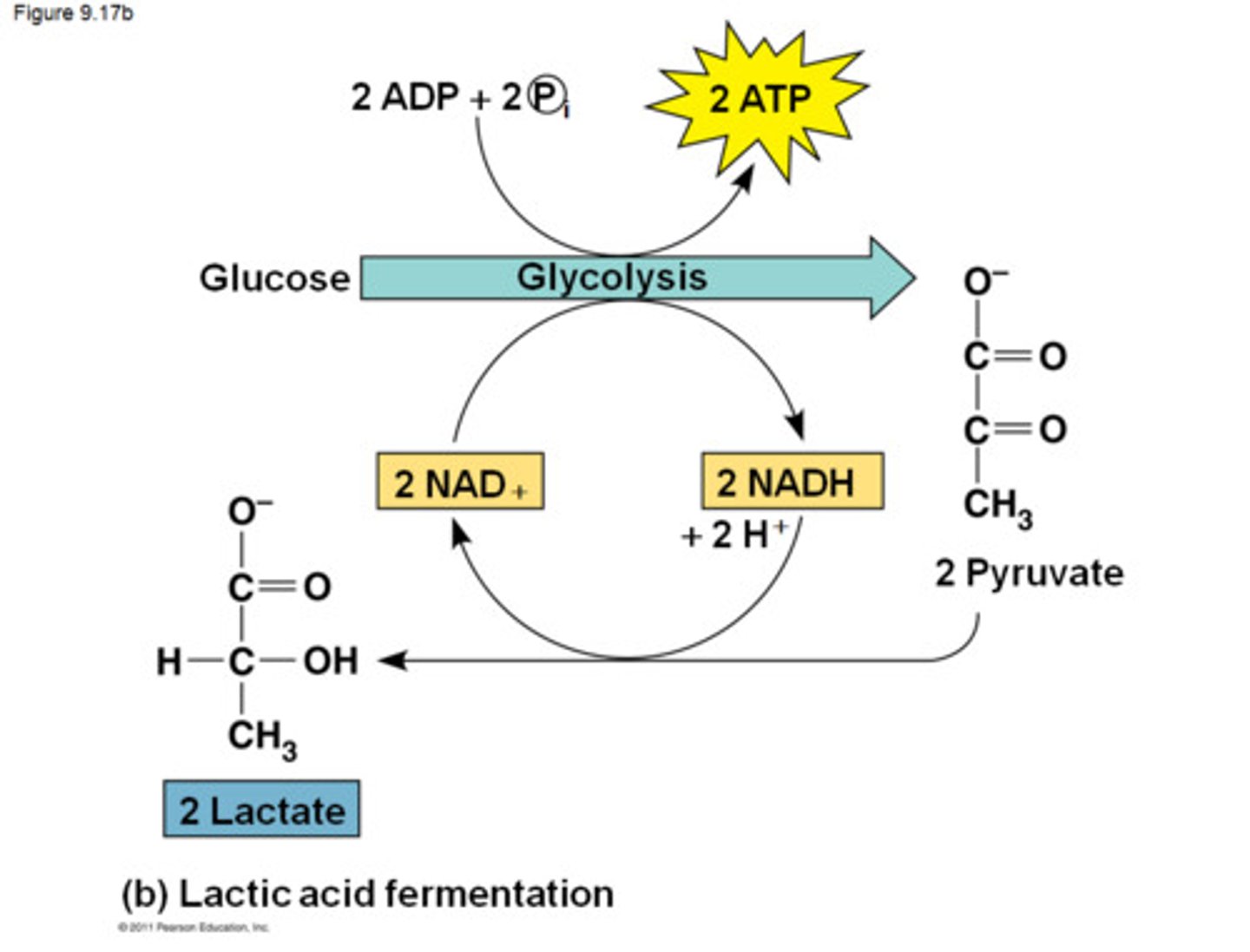
Pyruvate to Lactate
lactate dehydrogenase
NADH to NAD+
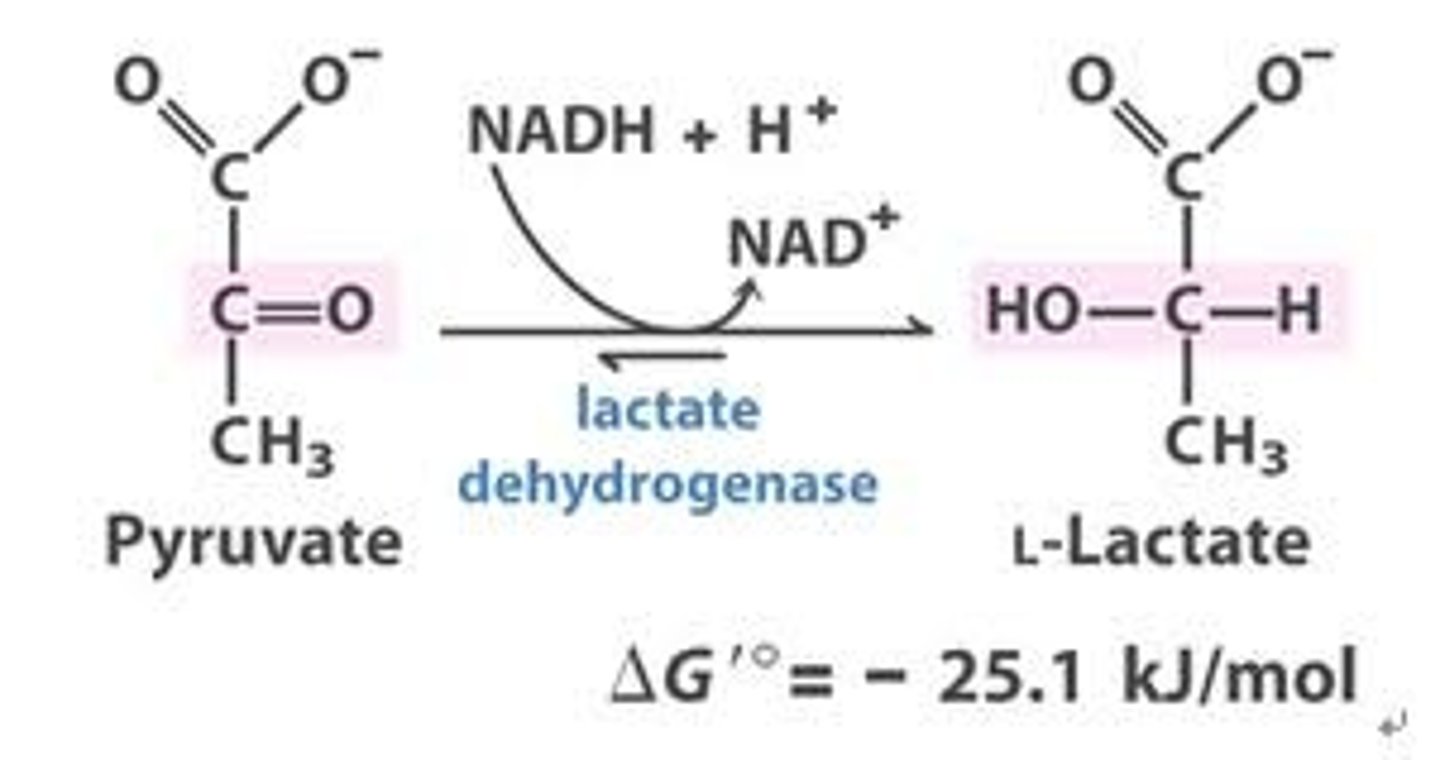
Depleted NAD+
stops glycolysis
pyruvate to ethanol
Pyruvate is catalyzed by pyruvate decarboxylase and H+ to acetaldehyde and CO2 which is then catalyzed by alcohol dehydrogenase and NADH to Ethanol and NAD+
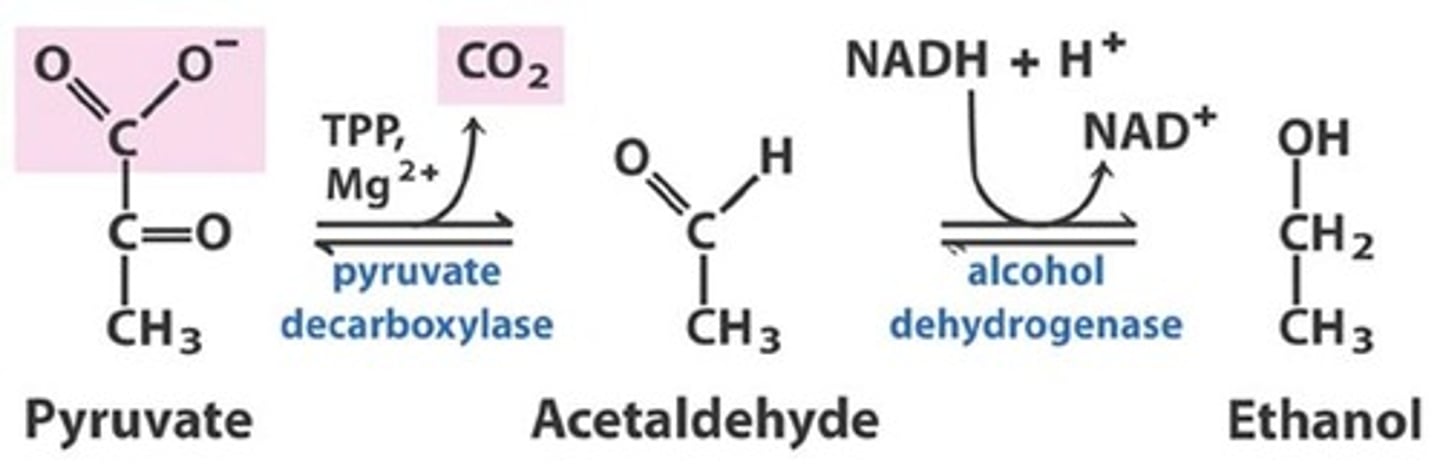
pyruvate dexarboxylation
only found in yeast cells
aerobic fate of pyruvate
pyruvate is oxidizied into Acetyl-CoA by pyurvate dehydrogenase complex and NADH and CO2 is produced
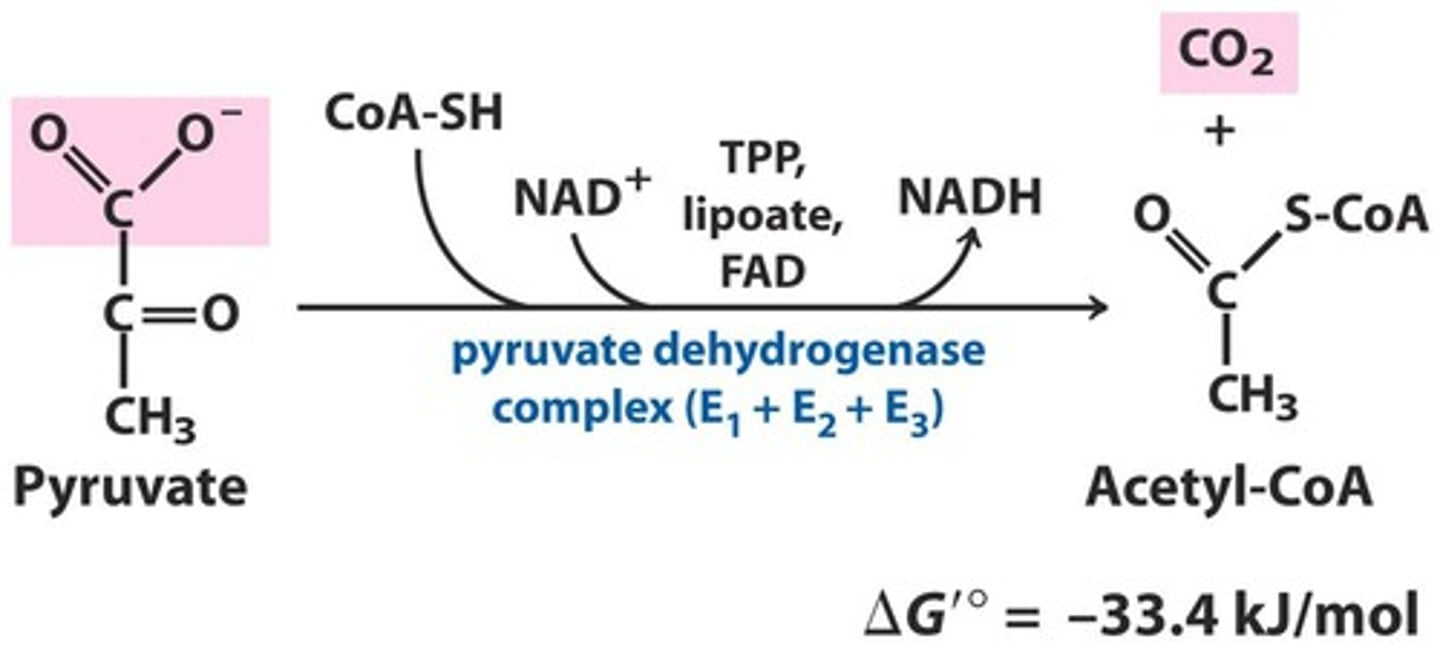
pyruvate dehydrogenase complex
enzymes that convert pyruvate into acetyl-CoA
E1.E2.E3 subunits
involved in regulation
reduced form
NADH and FADH2 (carries electrons)
thiolester bond
acetyl-CoA bond that holds energy
Dehydrogenase
An enzyme that catalyzes a chemical reaction during which one or more hydrogen atoms are removed from a molecule.
Performs redox reactions
more energy when fully oxidized
more C-C and C-H bonds (contain more energy)
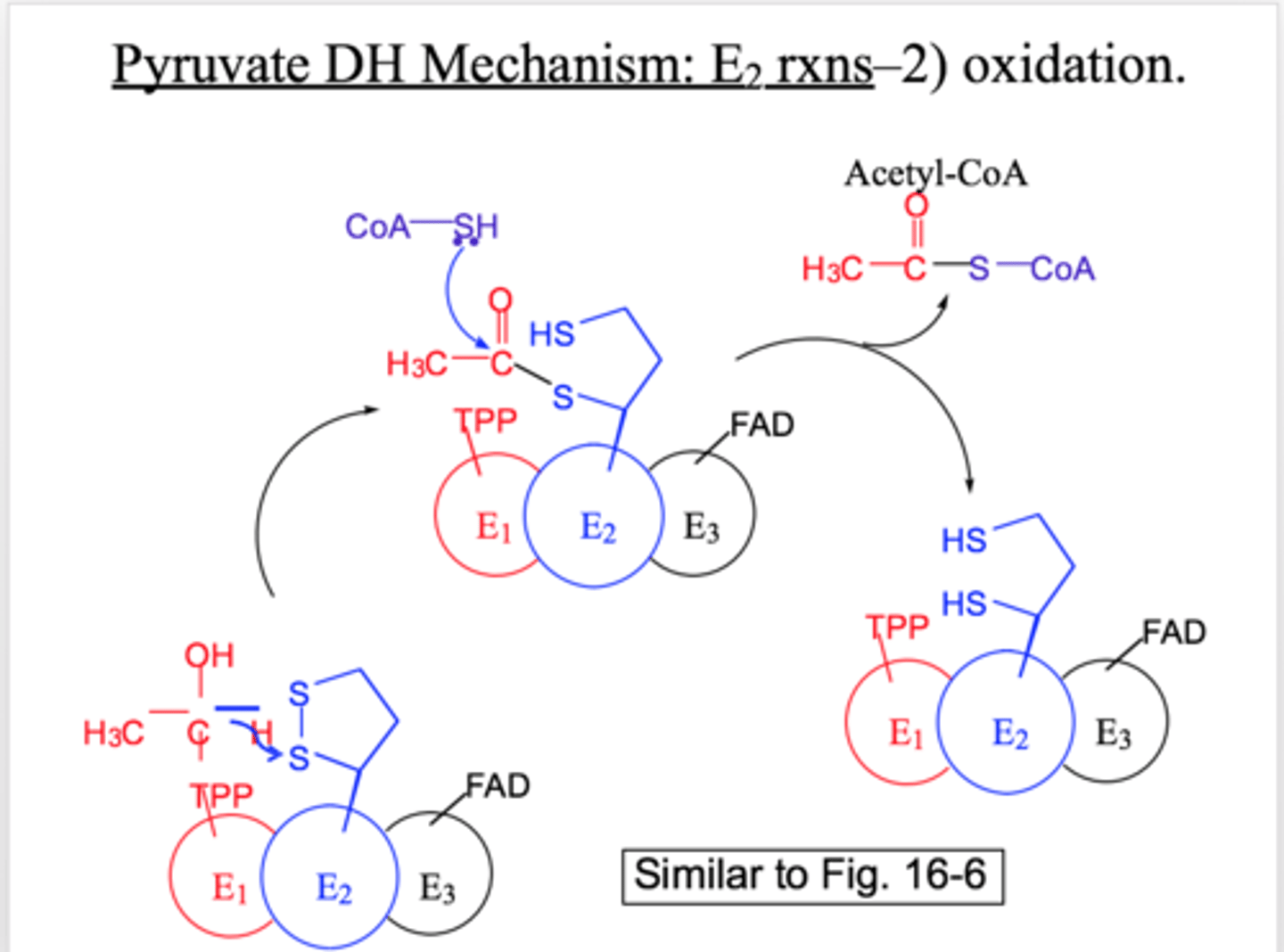
pyruvate DH: E2 reaction
oxidation
lipoic acid on site E2 gains e- and is reduced
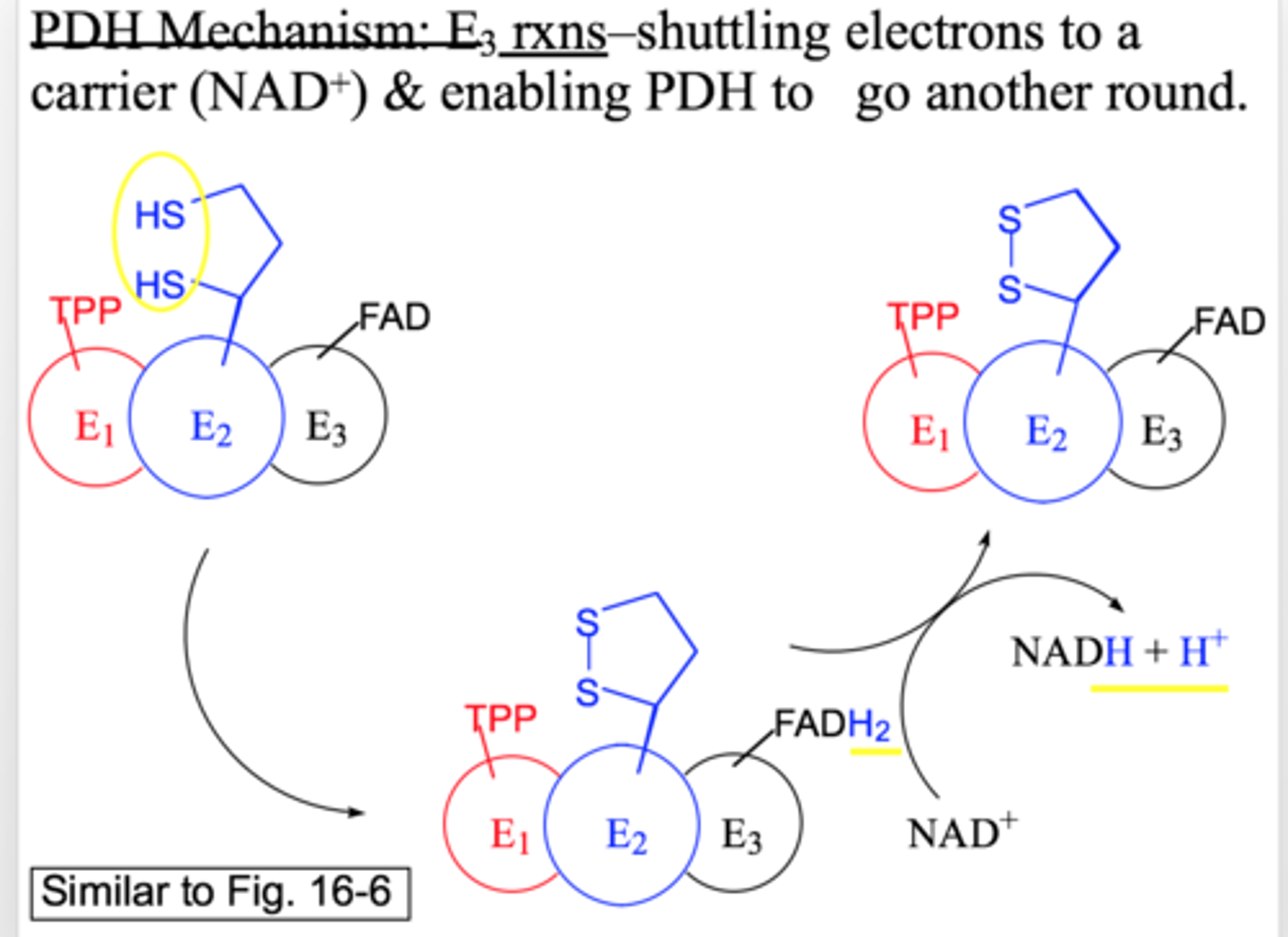
pyruvate DH: E3 reaction
electron shuttling
the e- transfer from lipoic acid to NAD+ which enables PDH to go another round
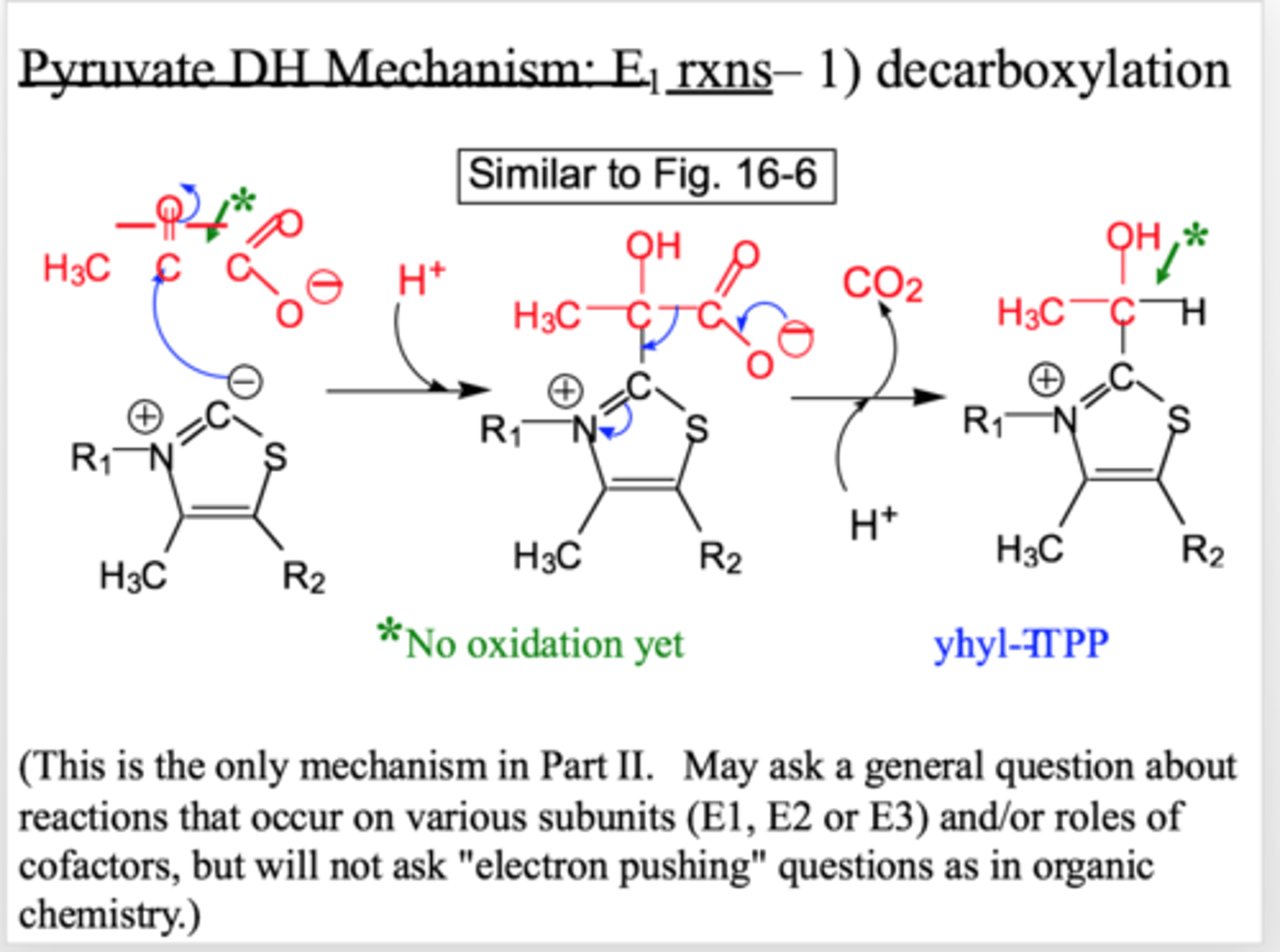
pyruvate DH: E1 reaction
decarboxylation
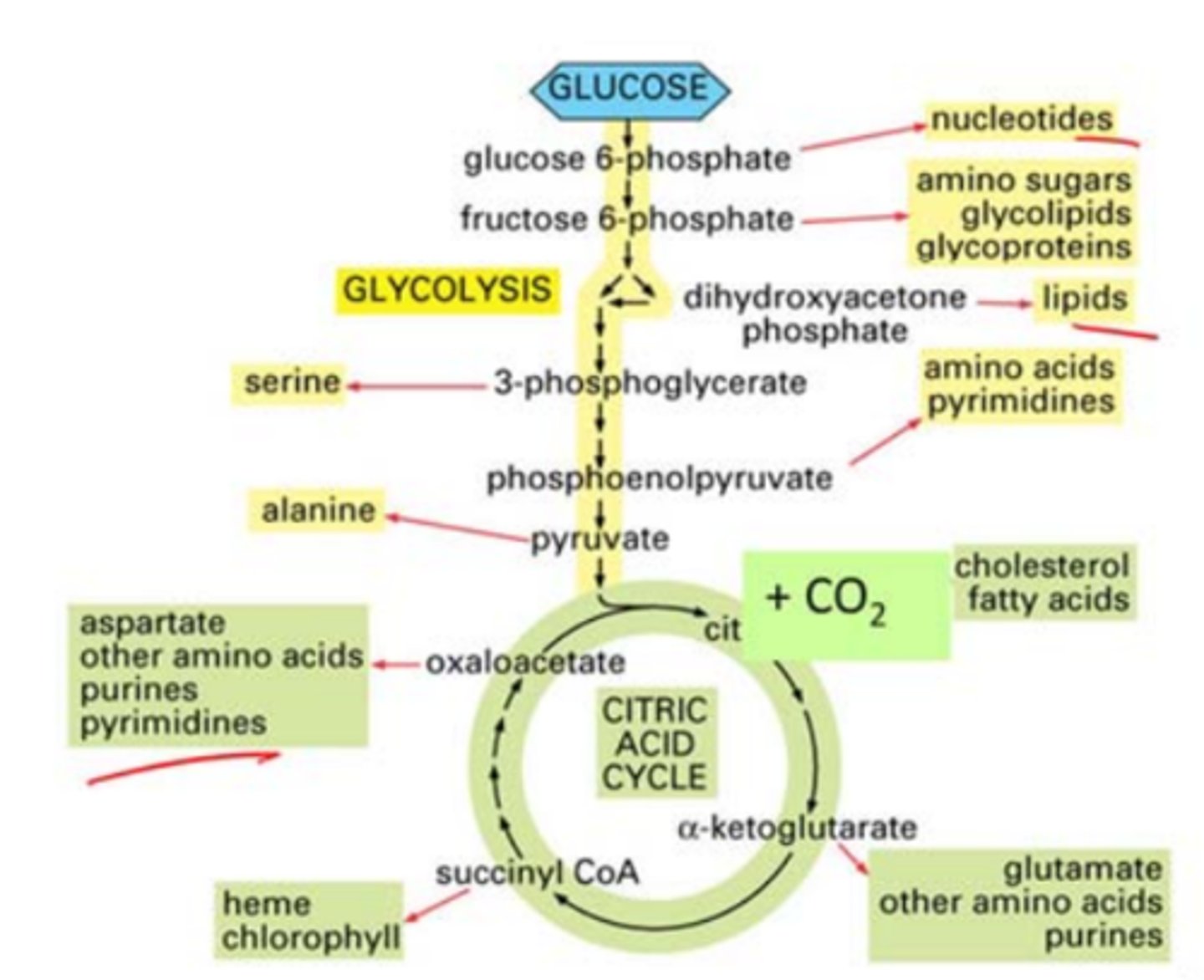
citric acid cylce
completes the breakdown of glucose
central point of lipid, carb, and protein breakdown
in mitochondria
intermediates recylced
CAC step 1
Citrate synthase adds an acetyl group ( 2 carbons) to oxaloacetate
Enzyme: citrate synthase
∆G= favorable (hydrolysis of Co-A bond)
IRREVERSIBLE
Co-A recycled cofactor
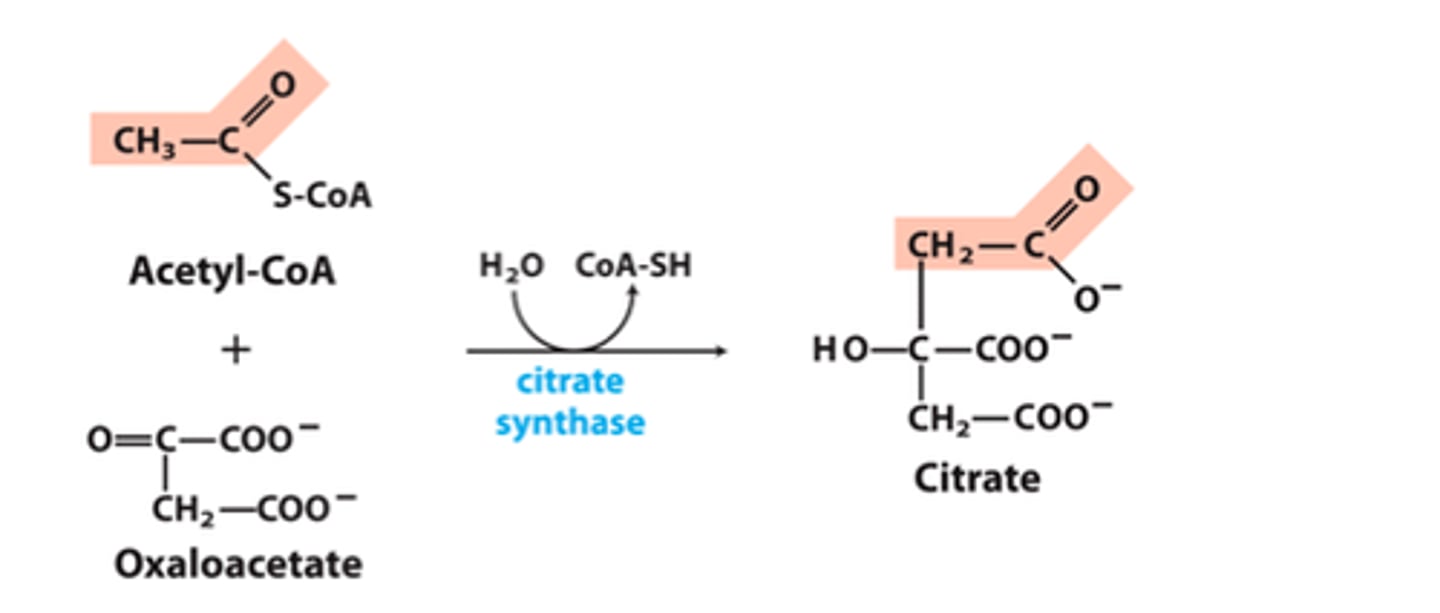
CAC step 2
Aconitase isomerizes citrate to isocitrate (C-OH bond moved)
Enzyme: aconitase
∆G= 0/+
Aconitate is intermediate between these 2
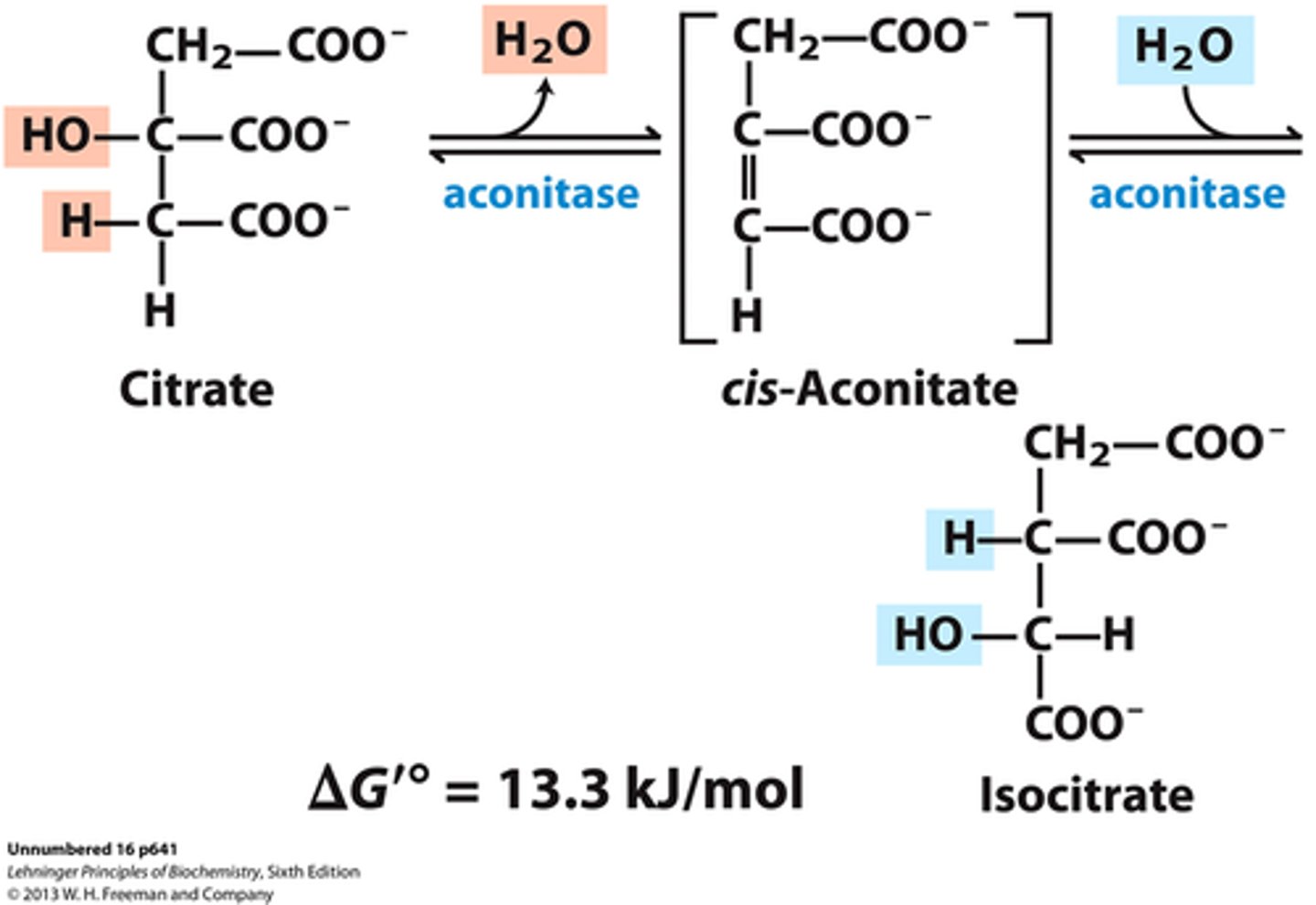
CAC step 3
Isocitrate dehydrogenase releases the first CO2
Enzyme: isocitrate dehydrogenase
∆G= favorable
IRREVERSIBLE
Isocitrate to α-ketoglutarate, make an NADH, and lose a CO2 (from oxaloacetate)
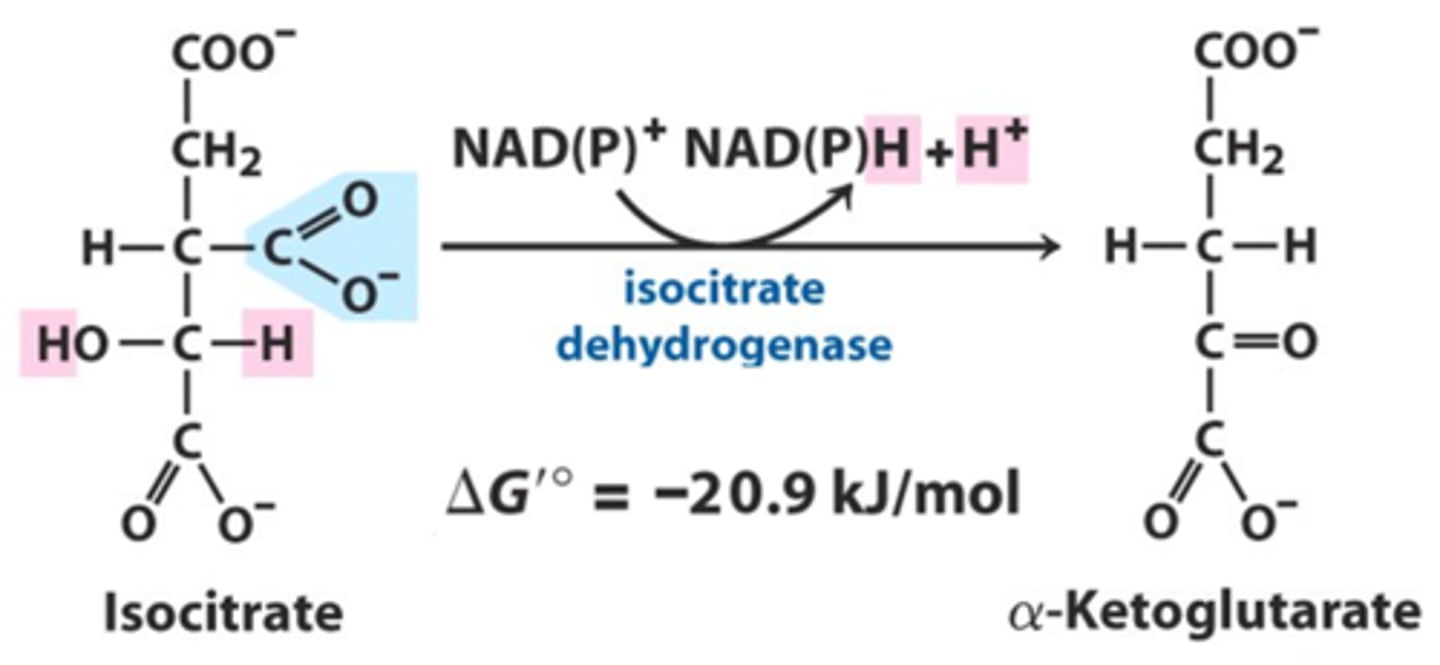
oxidative decarboxylation
COOCH into CO2
CAC step 4
α-Ketoglutarate dehydrogenase releases the second CO2
Enzyme: α-Ketoglutarate Dehydrogenase Complex (α-KDHC)
IRREVERSIBLE
LOSE CO2 and MAKE NADH
-CO2 came from oxaloacetate (has not lost acetyl group from acetyl-CoA)
Succinyl CoA
conserved energy in thioester in NADH
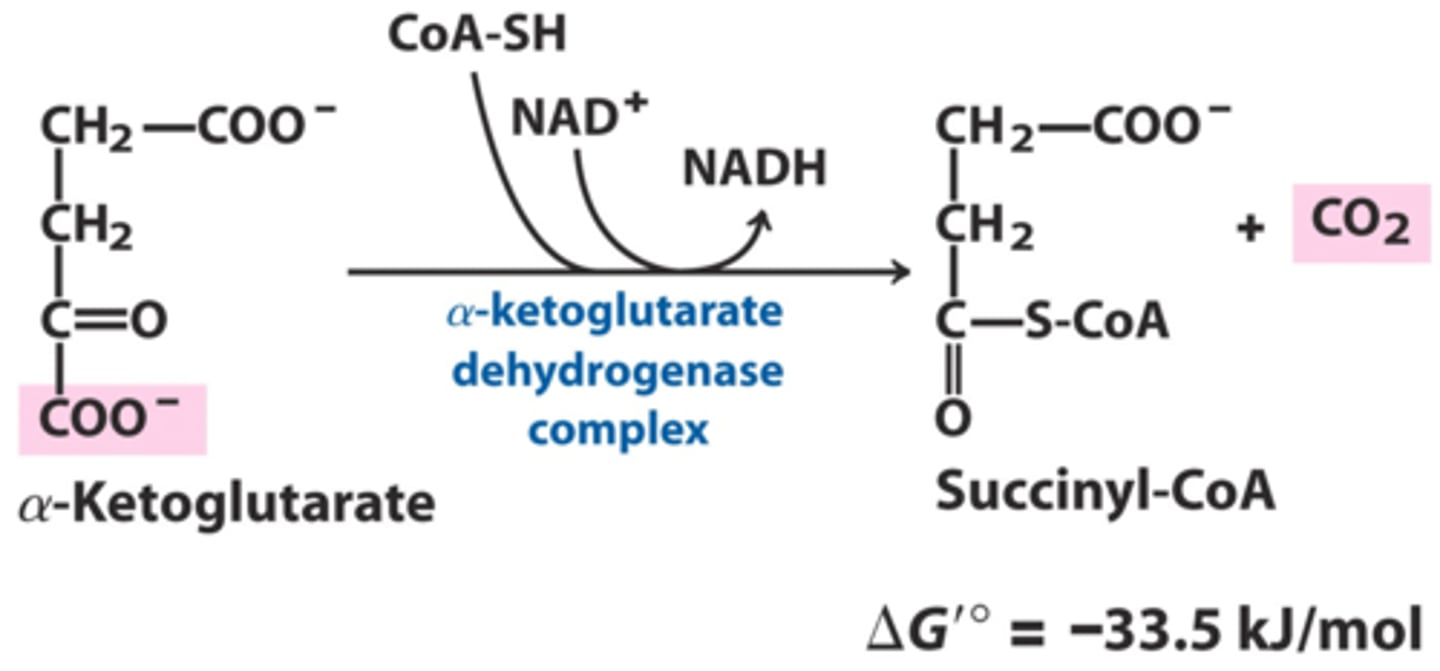
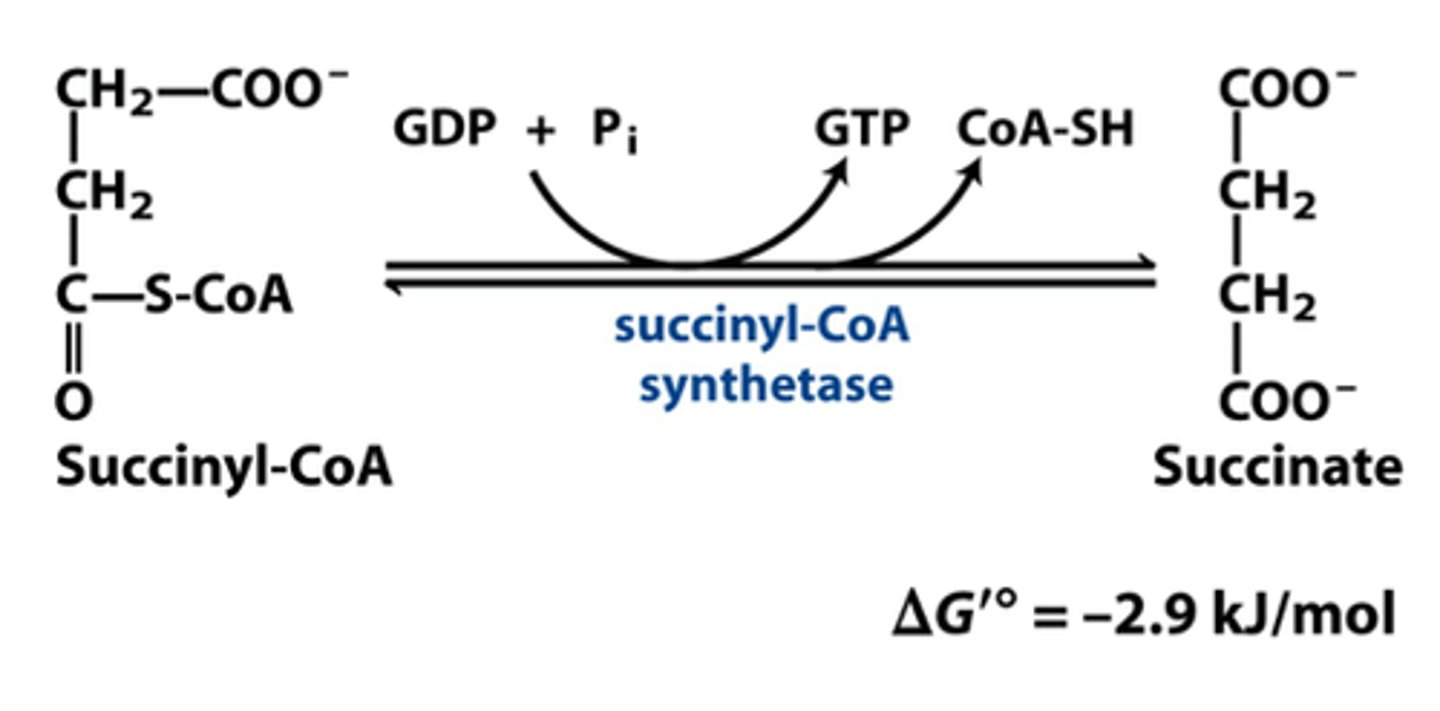
CAC step 5
Succinyl-CoA synthetase catalyzes substrate level phosphorylation
Enzyme: succinyl-CoA synthetase
Need to kick off CoA
1st substrate level phosphorylation
Make GTP
Take phosphate off a substrate and put it on GDP to make GTP
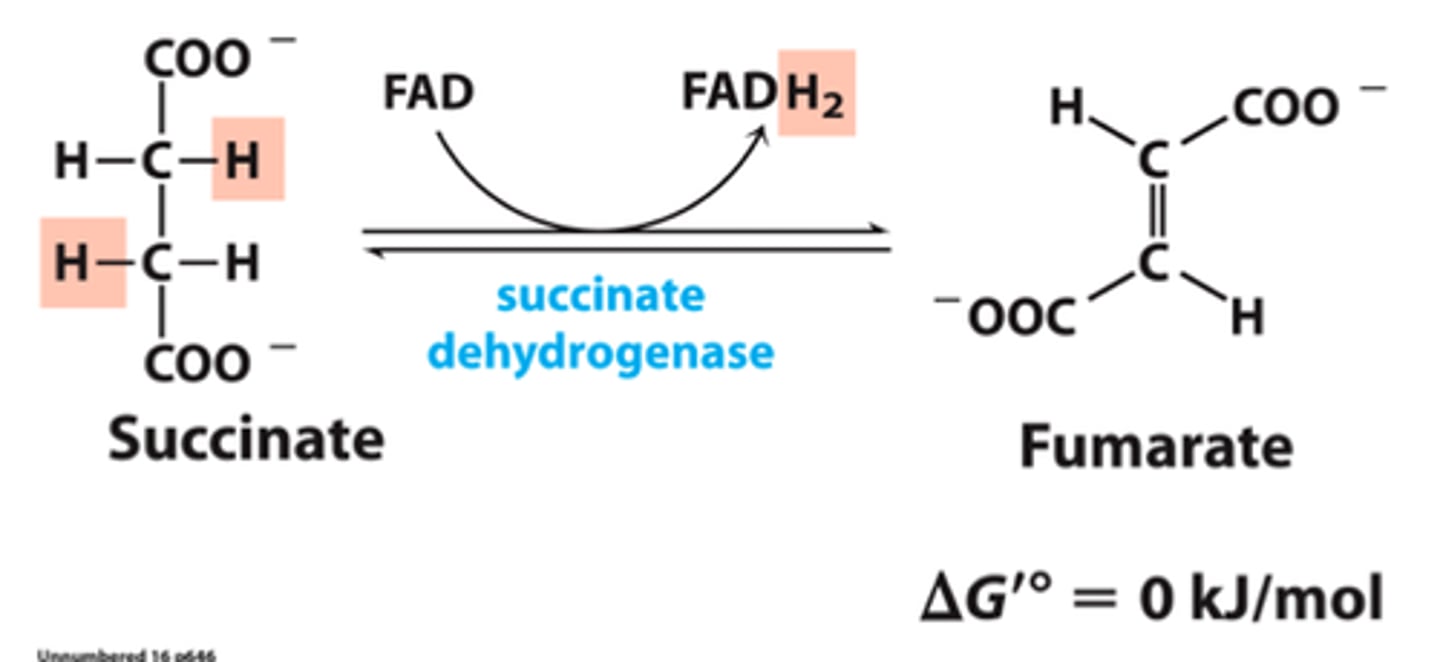
CAC step 6
Succinate dehydrogenase generates ubiquinol
Enzyme: Succinyl dehydrogenase
Enzyme participates in krebs cycle (TCA) and in electron transport (ETC)
Only enzyme that sits in INNER MEMBRANE of mitochondria (enzyme cannot move)
-(rest of enzymes float around in inner membrane space)
Succinate -> fumarate, make FADH2 -> passes e- to coenzyme Q
Electrons taken off of succinate added to FAD
FADH2 passes e- to coenzyme Q to make QH2 (very important for ETC)
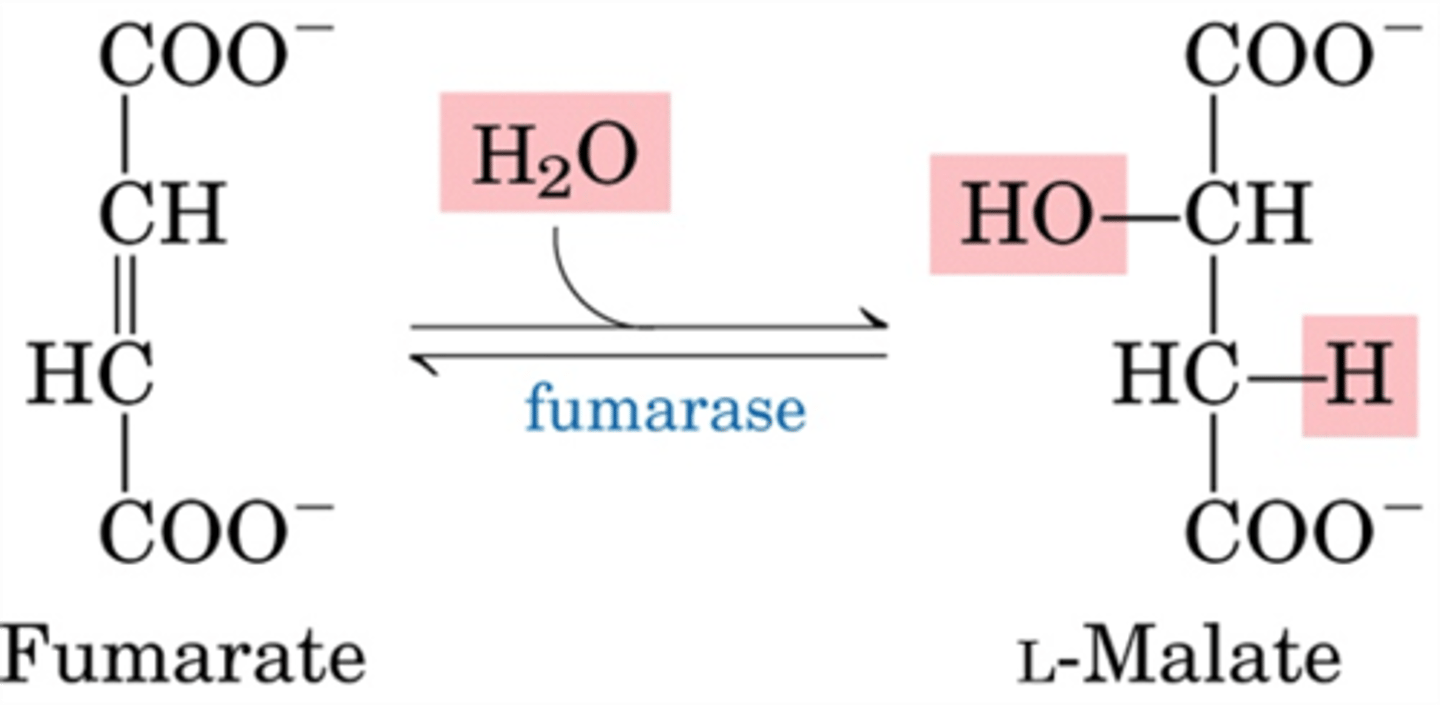
CAC step 7
Fumarase catalyzes a hydration reaction
Enzyme: fumarase
Fumarate -> malate (happens fast)
Fumarate considered catalytically perfect
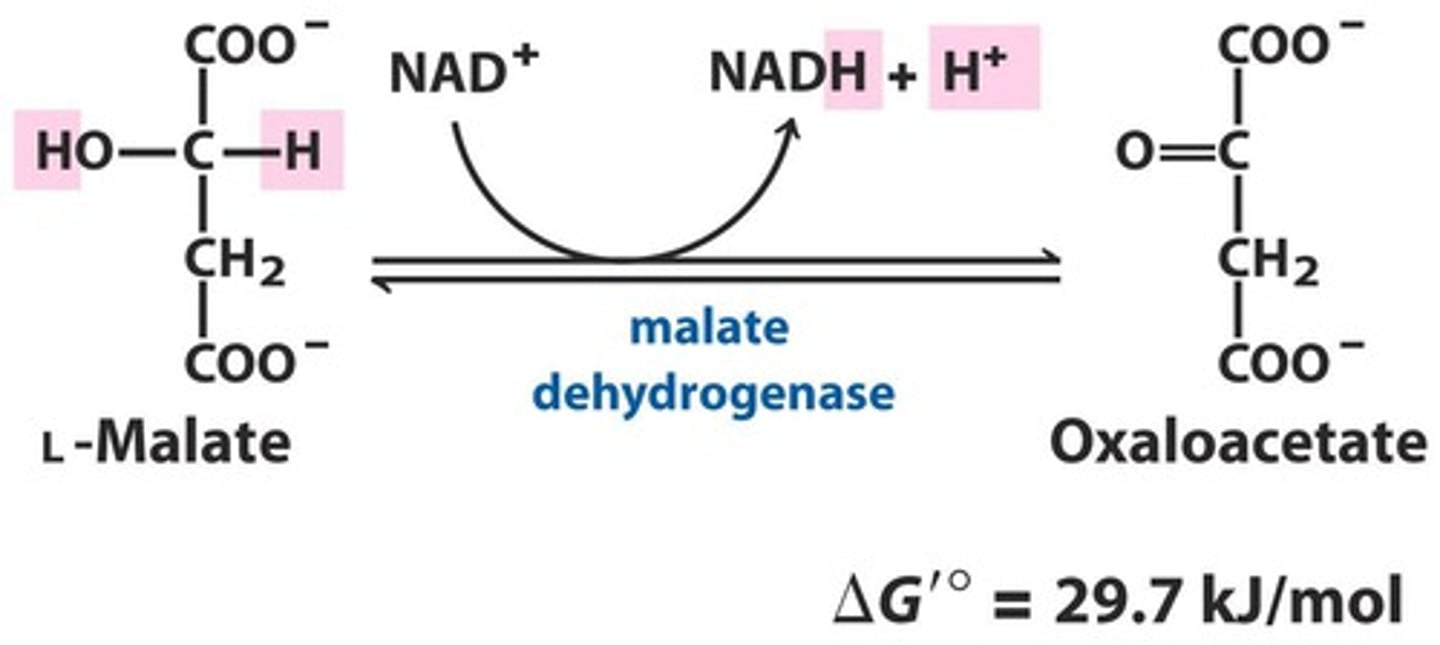
CAC step 8
Malate dehydrogenase regenerates oxaloacetate
Enzyme: malate dehydrogenase
Malate -> oxaloacetate, make NADH
After this goes back to step 1 and keeps going
-Highly endergonic +∆G= unfavorable
desaturation
C-C to C=C
C-H to C-C
(also oxidation)
regulation of the citric acid cylce
inhibitors: Increases energy (ex: atp produced)
stimulators: decreases energy (ex: amp, adp)
reactants in citric acid cylce
glucose/2pyruvate
NAD+ ,FAD, H20, ADP
products in citric acid cylcle
CO2, NADH, FADH2, ATP
replenishing anaplerotic reaction
pyruvate + HCO3- and ATP
adds CO2 to oxaloacetate
enzyme: pyruvate carboxylase (biotin)
Fatty acid oxidation
the metabolic breakdown of fatty acids to acetyl CoA; also called beta oxidation.
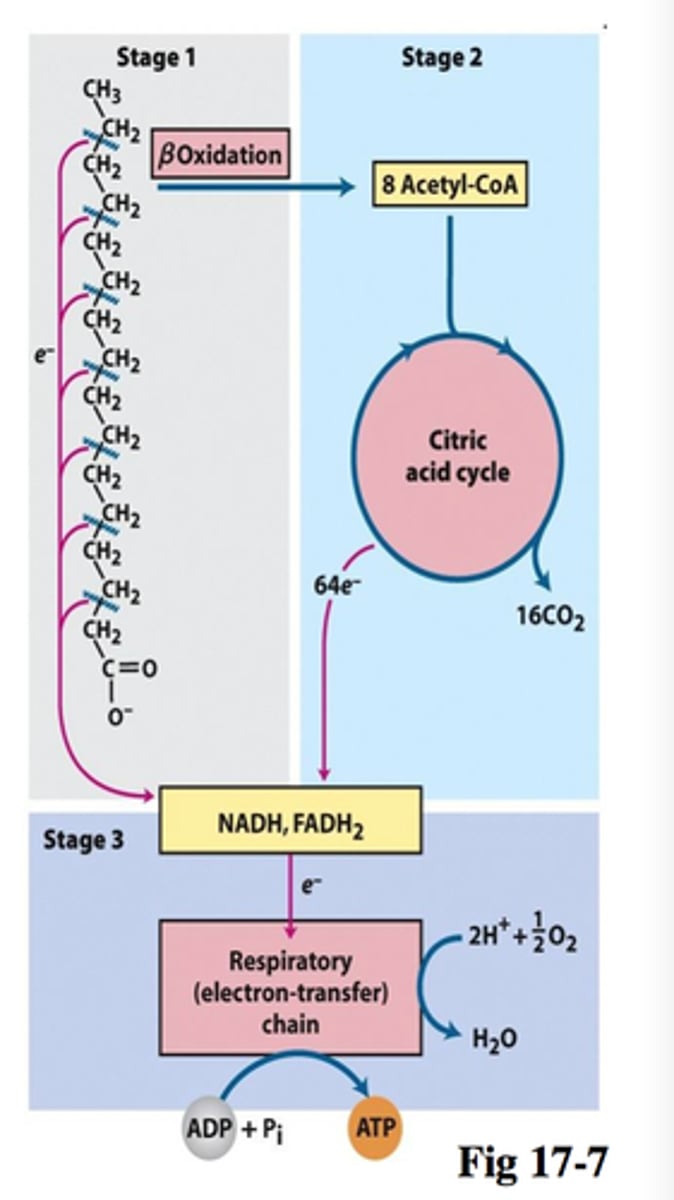
FA oxidation step 1
Activation: Fatty acid join to Co-A
-enzymes on outer mitochondrial membrane
FA oxidation step 2
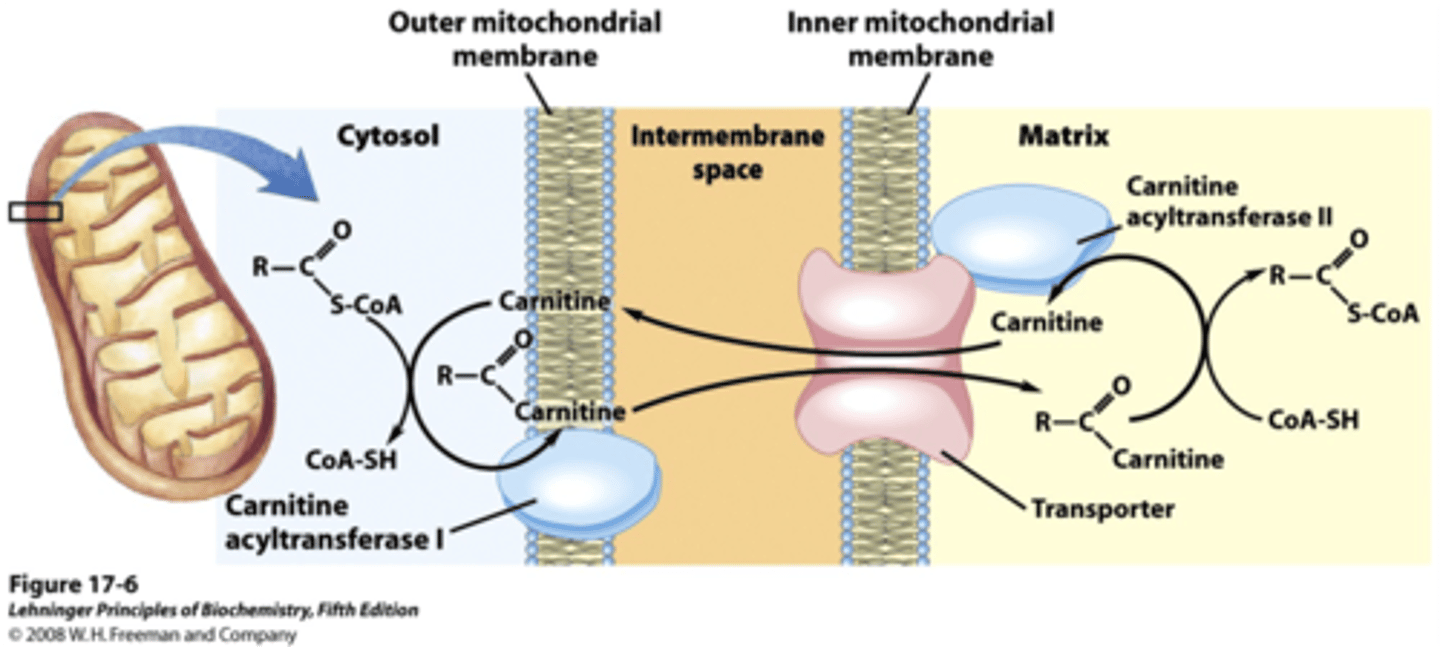
Transport: across inner mitochondrial membrane into mitochondrial matrix
-carnitine carrier system separates cytosolic and mitochondrial pools
FA oxidation step 3
Beta Oxidation: conversion of fatty acid into Co-A units in mitochondrial matrix
-Splitting fatty acid chain into 2-cabon sections
-4 step process
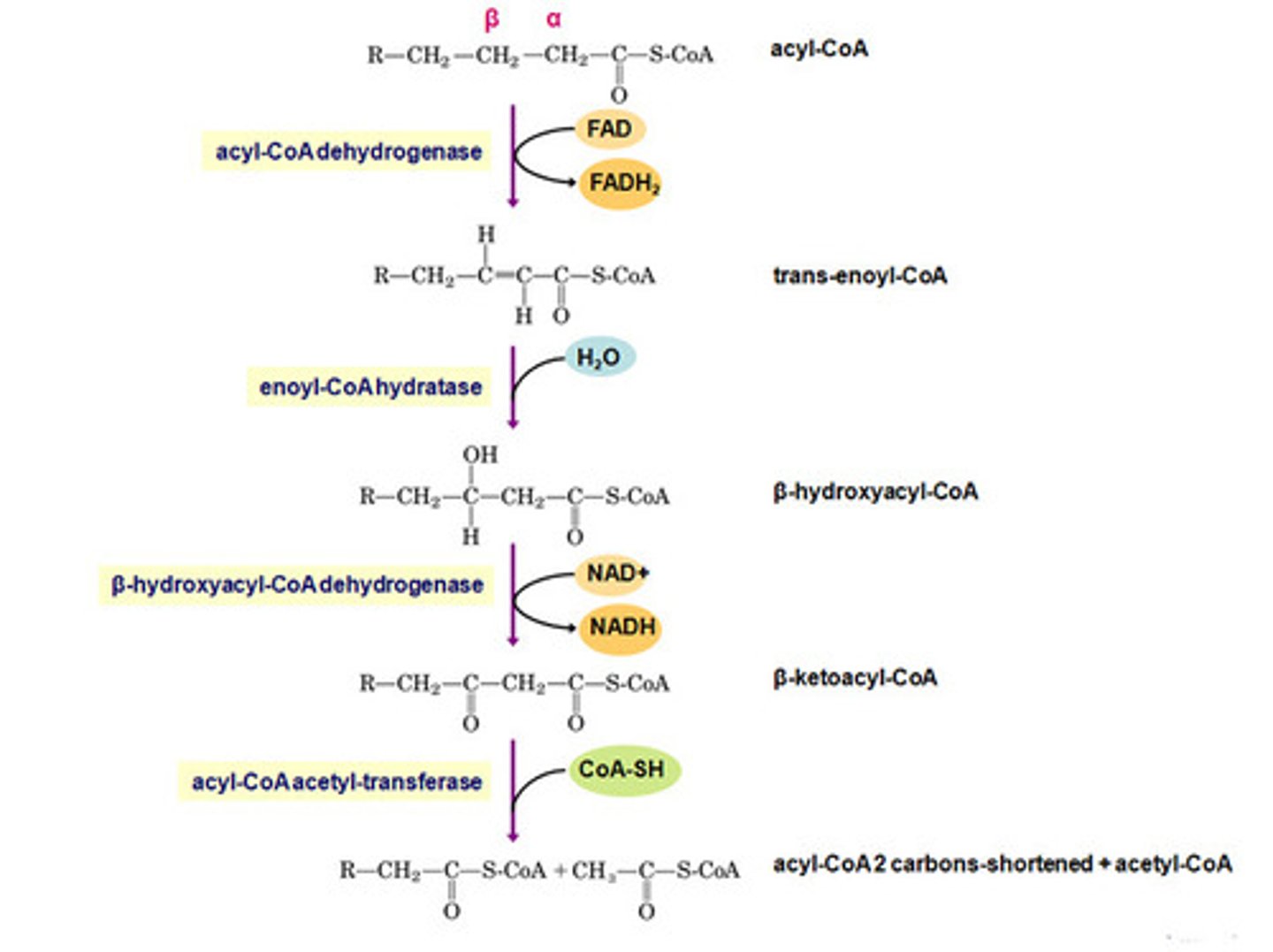
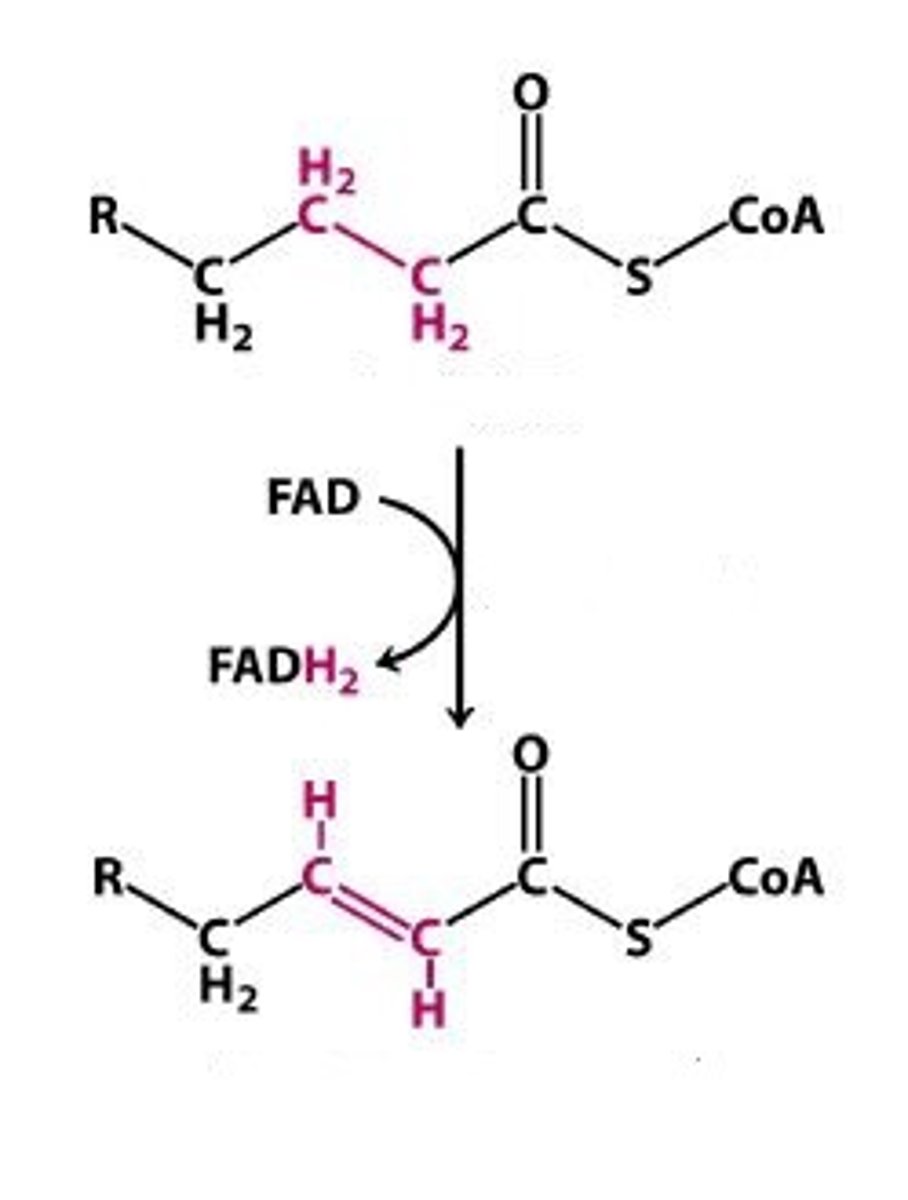
B-oxidation step 1
Dehydrogenation: C-H bonds into C=C bond
FAD to FADH2
Enzyme: Fatty Acyl-CoA dehydrogenases
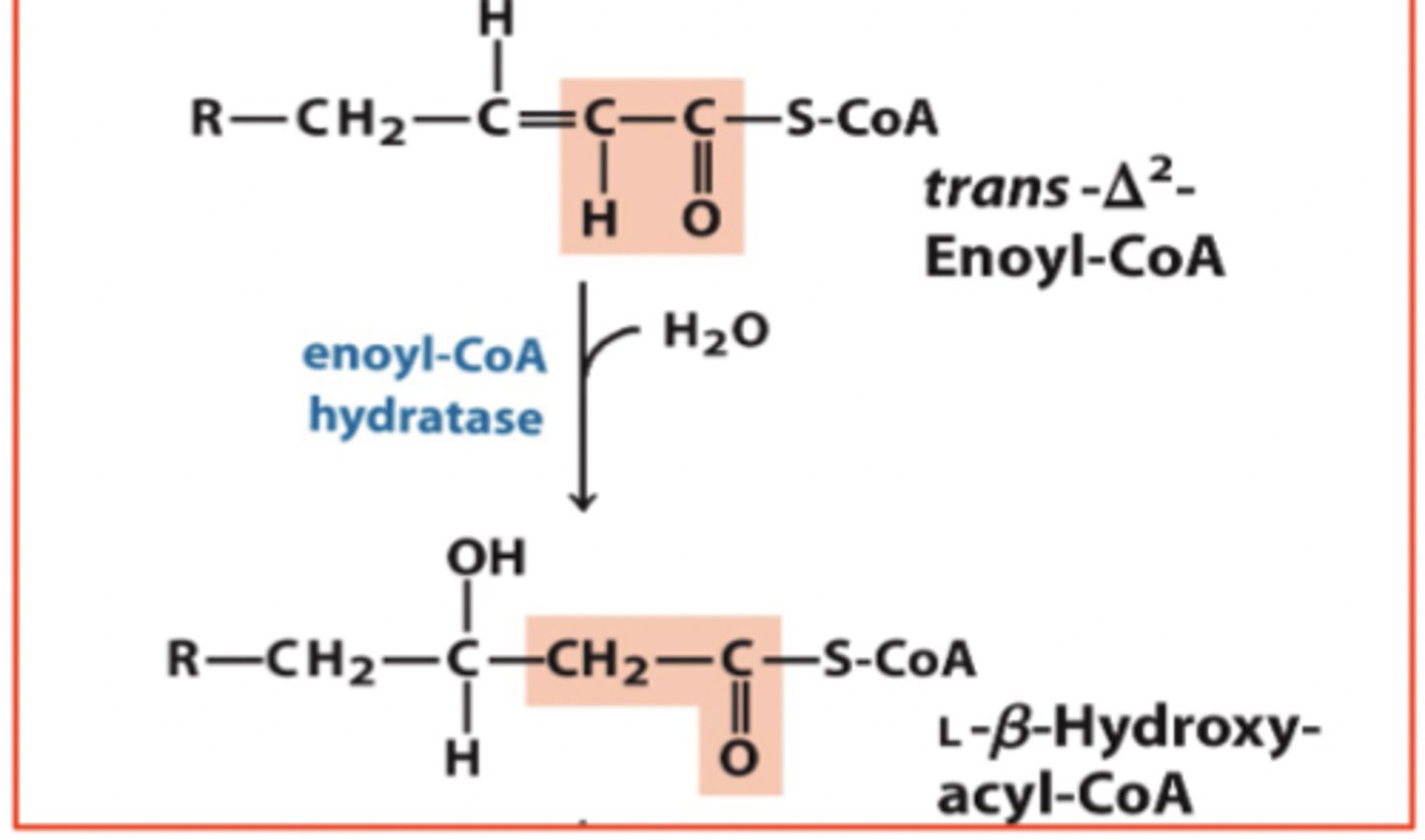
B-oxidation step 2
Hydration: C=C bond to C-OH and C-H bond
addition of H2O
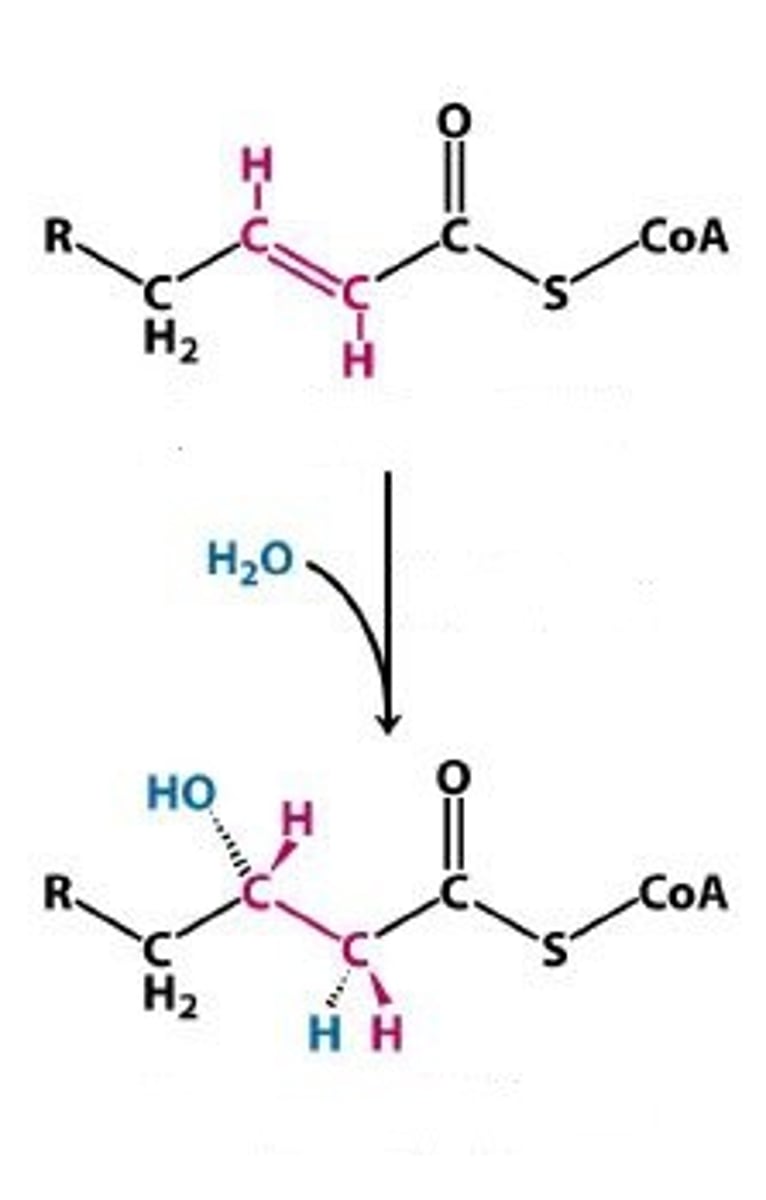
B-oxidation step 3
dehydration again
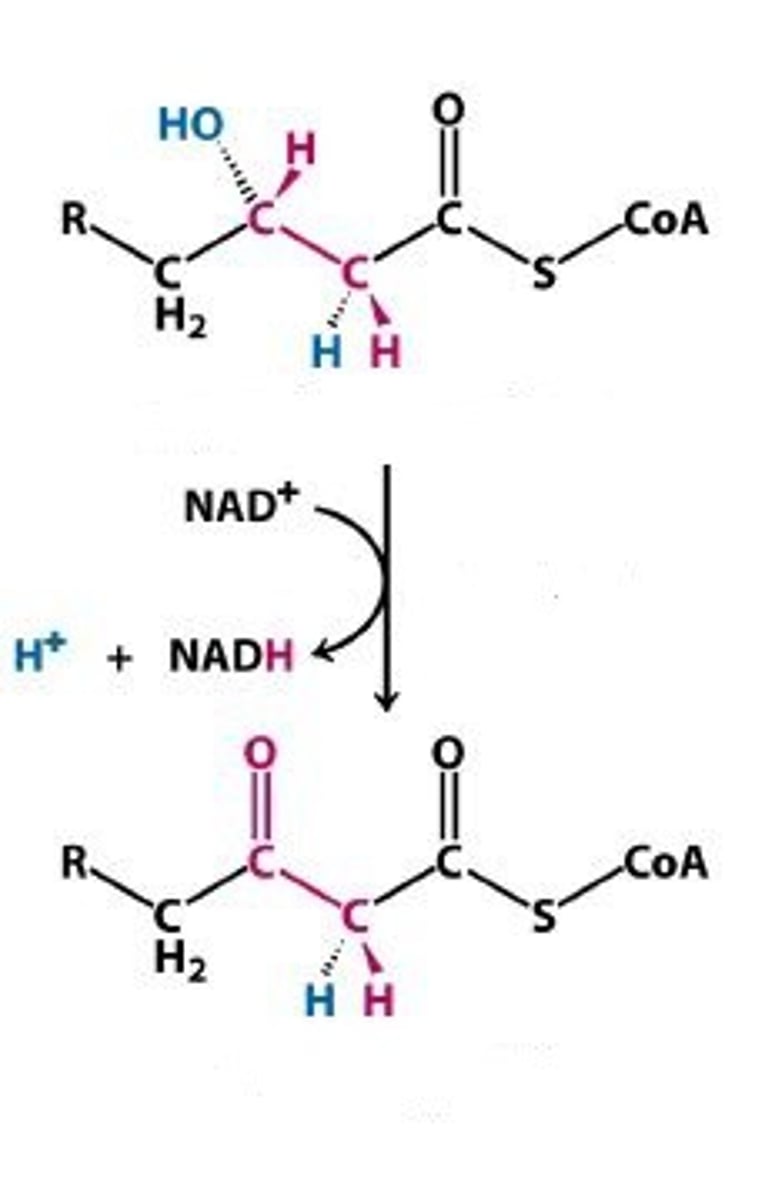
B-oxidation step 3
Thiolytic cleavage: the formation of the 2-carbon sections formed and the s-CoA is bonded.
overall B-oxidation
8 acetyl-CoA + 7 FADH2 + 7NADH
# of acetyl-CoA
n-1 B-oxidations
(n= # of 2C-atom segments)
Fatty acids in mitochondria
in mitochondria: fatty acids broken down
Cytosol: fatty acids produced in excess carbohydrates
ETC keeps pools separate
Malonyl CoA
Fatty Acid Metabolism
Acts as an inhibitor to Carnitine Acyltransferase I (CAT-1)
also known as Carnitine Palmitoyl Transferase I
Thus preventing the transport of Fatty-Acyl-CoA via the Carnitine shuttle to the mitochondria.
Fattay acyl Co-A
cannot be brought into matrix alone, must be converted into carnitine F.A. and brought into matrix and converted back into fatty acyl Co-A
carnitine
a small, organic compound that transports free fatty acids from the cytosol into the mitochondria for oxidation
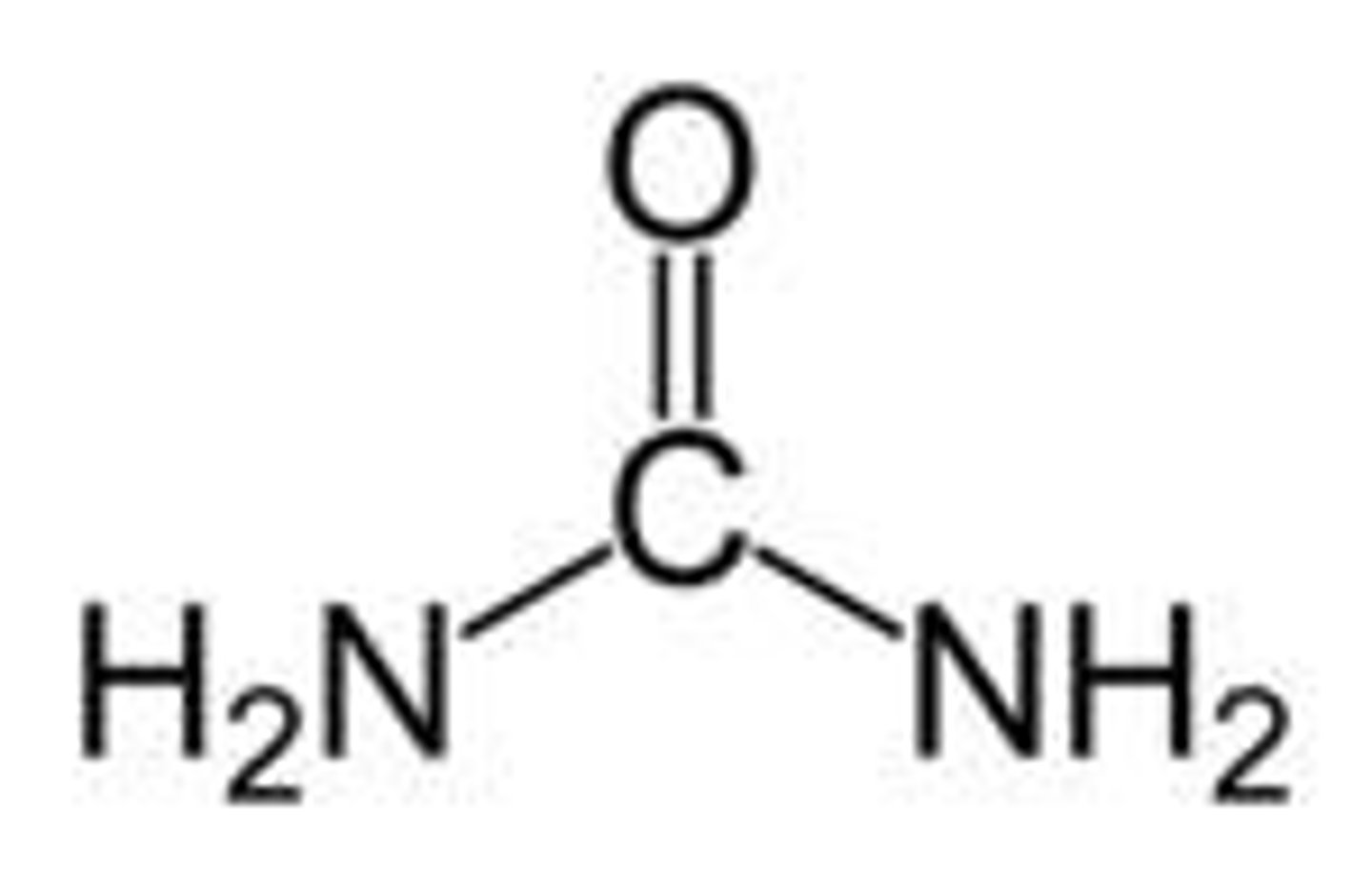
products of burning proteins
CO2 + H2O + (NH4+)
NH4+ elimination
N-atoms from amino groups are attached to CO2 through urea cycle to produce urea (waste)
detoxification
glutamine synthetase adds NH3 to glutamate to glutamine
extrahepatic
NH4+ is detoxified to liver as glutamine which will not diffuse to the brain
removal of amino group
Keto acids produced by transaminations which are used for CAC for gluconeogenesis and glutamate for liver
transaminations
swapping amino groups and catalzyed by aminotransferases
aminotransferases
transfers amino groups and turns glutamate (amino acids) --> alpha-ketoglutarate
alanine aminotransferase
Converts Pyruvate to Alanine (a-ketogluatate to glutamate) and is highly reversible (Cycling)
keto acid
ketone with a COO- attached
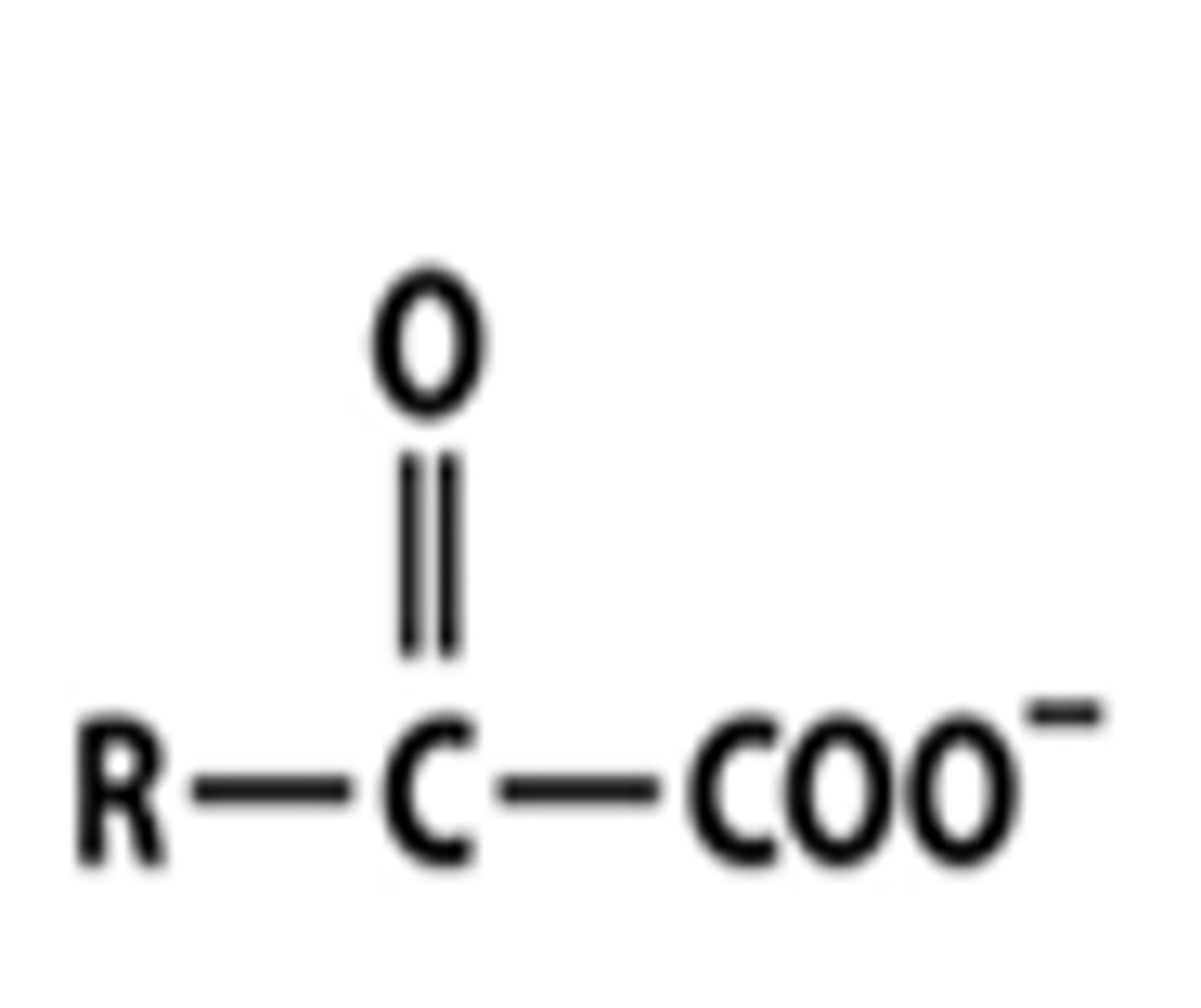
N-atoms flow in urea cylce
1) urea via amino acids to glutamine + glutamate
2) NH4 to aspartate
N-donor
glutamine and glutamate in cytosol give up their N-atoms to mitochondrial liver
excess amino acids and alanine
end in liver as glutamate via a-ketoglutarates and a-keto acid cylcing (outside mitochondria)
how does NH4+ form
glutamate/mine form NH4+ by glutaminase/glutamate dehydrogenase
Glu to aspartate by asp aminotransferase
carbamoyl phosphate
fuel for the urea cycle (uses ATP, HCO3-, and NH4+)
Urea cycle step 1
ornithine + carbamoyl phosphate --> citrulline
Urea cycle step 2
Citrulline +Asparate -> Argininosuccinate
2 N-atoms for urea joined to C-atom of CO2
ATP used
energize/activate citrulline to react with aspartate
Urea cylce step 3
urea released by arignase
NH4+ and aspartate
provide N-atoms for urea production
ketogenic
amino acids converted to acetate
gluconeogenic
amino acids converted to CAC intermediate
oxidative phosphorylation
NADH and FADH2 are oxidized and produces ATP
reducing power
electrons available in NADH and FADH2 are transported to the ETC to O2 (makes H2O) and ATP
Reduction potential
Eº - affinity for e's (tendency to reduce/oxidize)
difference in reduction potentials
Delta Eº - electron motive force, "energy in redox reaction"
Delta Eº = (Lower Eº) - (higher Eº)
more positive Eº
gains electrons (reduced) and oxidized state has higher e- affinity
more negative Eº
loses electrons (oxidized) and oxidized state has lower e- affinity
NADH & H20
are reduced and NADH (electron donor)
O2 & NAD+
are oxidized and NAD+ (electron acceptor)
spontaneous direction
neg G change and positive E change
Cytochrome C
protein that shuttles electrons between complexes III and IV (associated with respirasomes)
Path of energy flow
electron-motive force (reducing power) is converted to a proton-motive force (proton gradient) and then into high energy phosphate bonds (ATP)
ETC pathway 1
NADH (electrons) to I --> Q --> III ---> Cyt C --> IV --> O2
ETC pathway 2
Succinate/FADH2 (electrons) to II ---> Q --> III ---> Cyt C ---> IV ---> O2
Respirasome
moves electrons from ubiquinol (QH2) to oxygen
ubiquinone
Coenzyme Q that is a soluble membrane mobile carrier
Complex 1
NADH-ubiquinone oxidoreductase (NADH dehydrogenase)
- catalizes oxidation of NADH and reduction of UQ helps pump 4H+
Complex 2
Succinate dehydrogenase
- catalyzes the oxidation of succinate and reduction of Ubq
- only membrane inserted enzyme of citric acid cycle
- no pumping of H+ (increases pool of ubiquinol)
Complex 3
Ubiquinone: cytochrome c oxidoreductase
-oxidizes ubiquinol and reduces Cyt C1
-pumps 4H+ per 2 e- transferred 2Cyt C1
Complex 4
cytochrome c oxidase
- complex where O2 is consumed
- Accumulates 4e- from 4 Cyt C and reduces O2 = H2O
- pumps 2H+ per 2e- / 4H+ per O2
- evolved to prevent the release of toxic partially reduced oxygen species
defense against reactive O2
reducing powers (NADPH) used to make glutamine that reduces enzymes. These enzymes then get rid of toxic by-products from O2 reactions