chem 215
1/91
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
92 Terms
CrO3, H2SO4, H20 (Jones Reagent)- 1 prime alcohol
carboxylic acid
CrO3, H2SO4, H20 (Jones Reagent)- 2 prime alcohol
ketone
CrO3, H2SO4, H20 (Jones Reagent)- 3 prime alcohol
cant oxidize
CrO3 and pyridine / ClCro3 - and pyridine+ --- 1 prime alcohol
aldehyde
CrO3 and pyridine / ClCro3 - and pyridine+ --- 2 prime alcohol
ketone
CrO3 and pyridine / ClCro3 - and pyridine+ --- 3 prime alcohol
can't react
Swern Oxidation- 1 prime alcohol
aldehyde
Swern Oxidation- 2 prime alcohol
ketone
Swern Oxidation- 3 prime alcohol
can't react
Swern Mechanism
-o reaches out to positive s
-cl group leaves
-pyridine deprotonates ch3 group on s
-intramolecular grab of h, which causes c=o to form and s to leave
socl2
cl switches with oh group, same stereo
socl2 w pyridine
cl switches with oh group, flips stereo
pcl3
cl switches with oh group, flips stereo
pbr3
br switches with oh group, flips stereo
pi3
i switches with oh group, flips stereo
epoxide opening to less substituted side
strong nucleophile, basic conditions
ex: 1. NaOCH3 2. H3O+
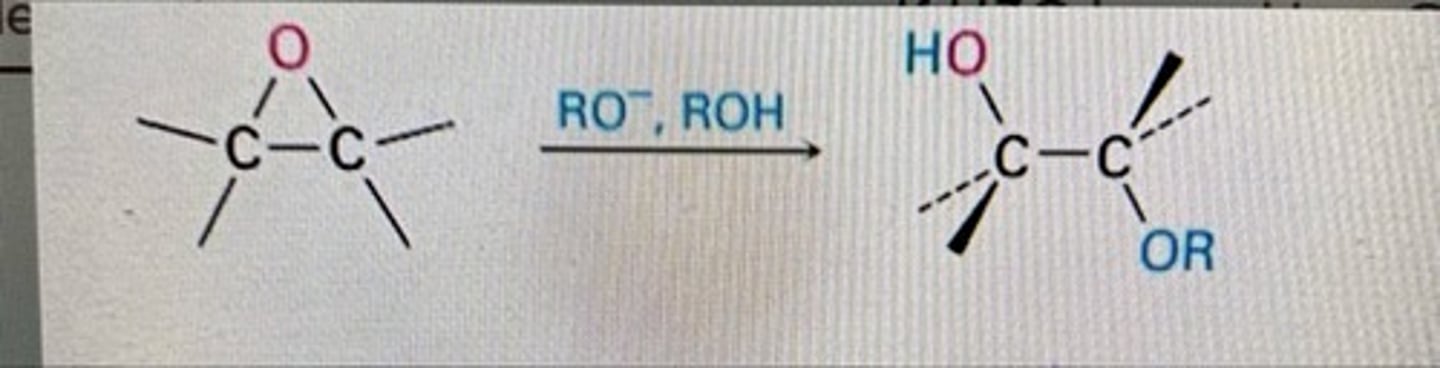
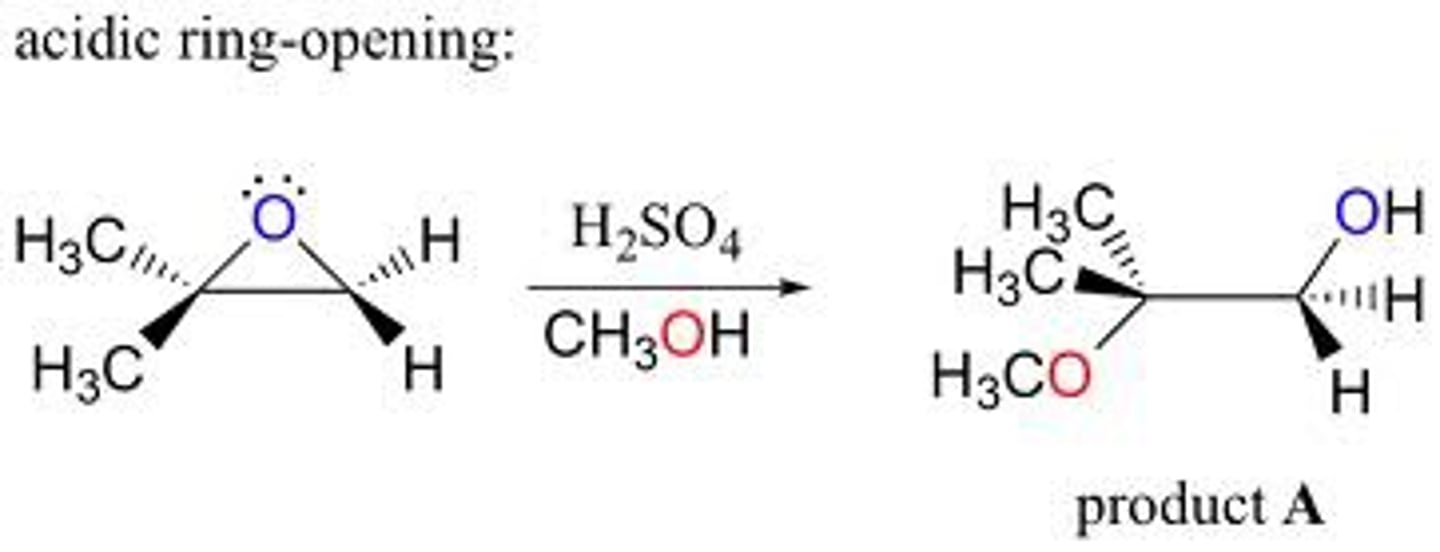
epoxide opening to more substituted side
weak nucleophile, acidic conditions
ex: HOCH3 + H2SO4
Strong nuc examples
LiAlH4, NaBH4, CICh3, MgBrCH3
weak nuc
ketals, acetals, mines
Organometallics/grignard
often attacks c=o bonds and makes carbon great nucleophile, halogen switch
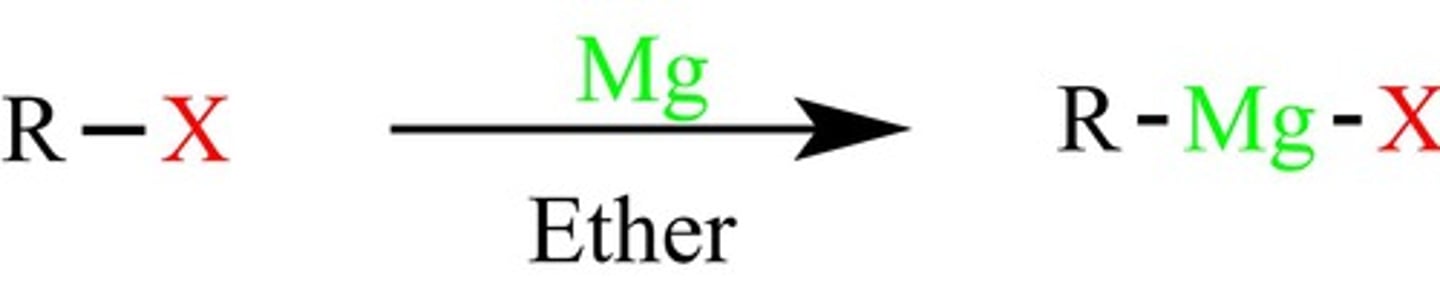
Grignard
mg, make carbon negative
organolithium
2 li, make carbon negative
hydride donors
LiAlH4, NaBH4- add h to c=o bond and with workup o becomes oh
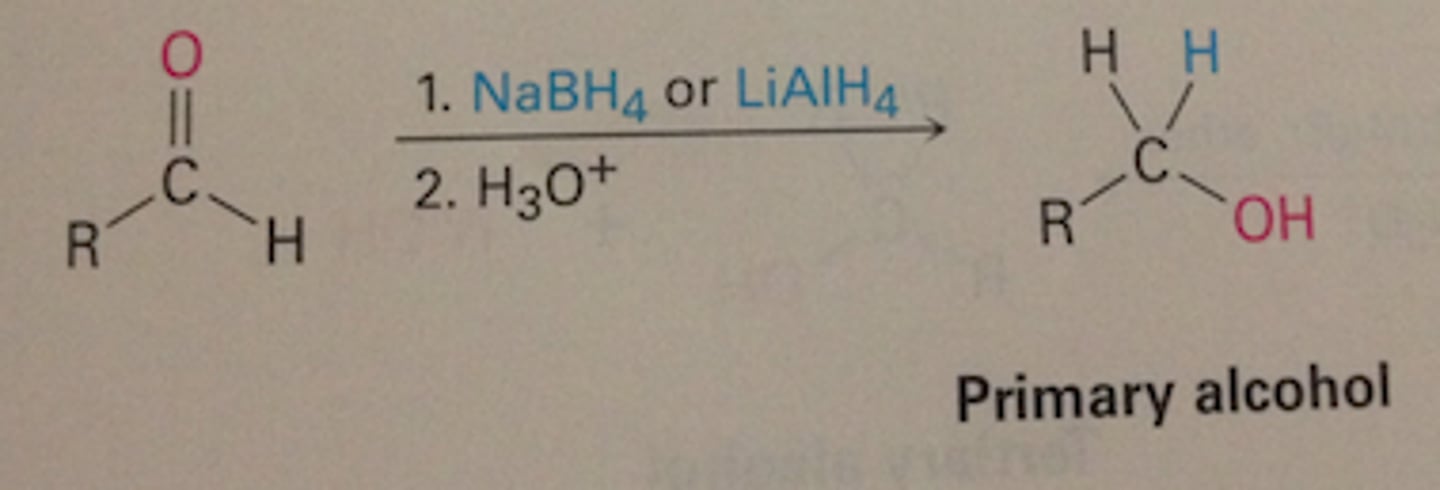
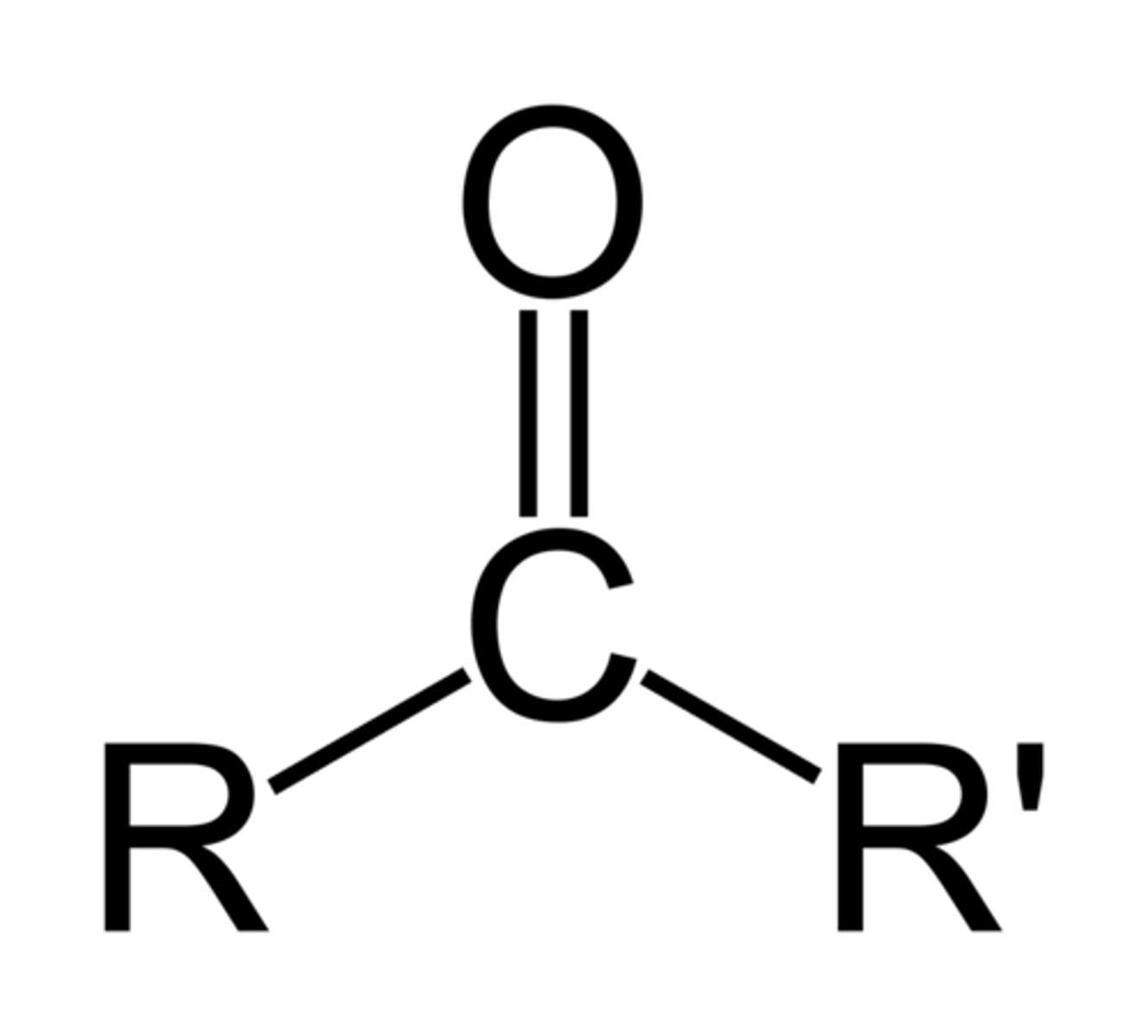
Ketone
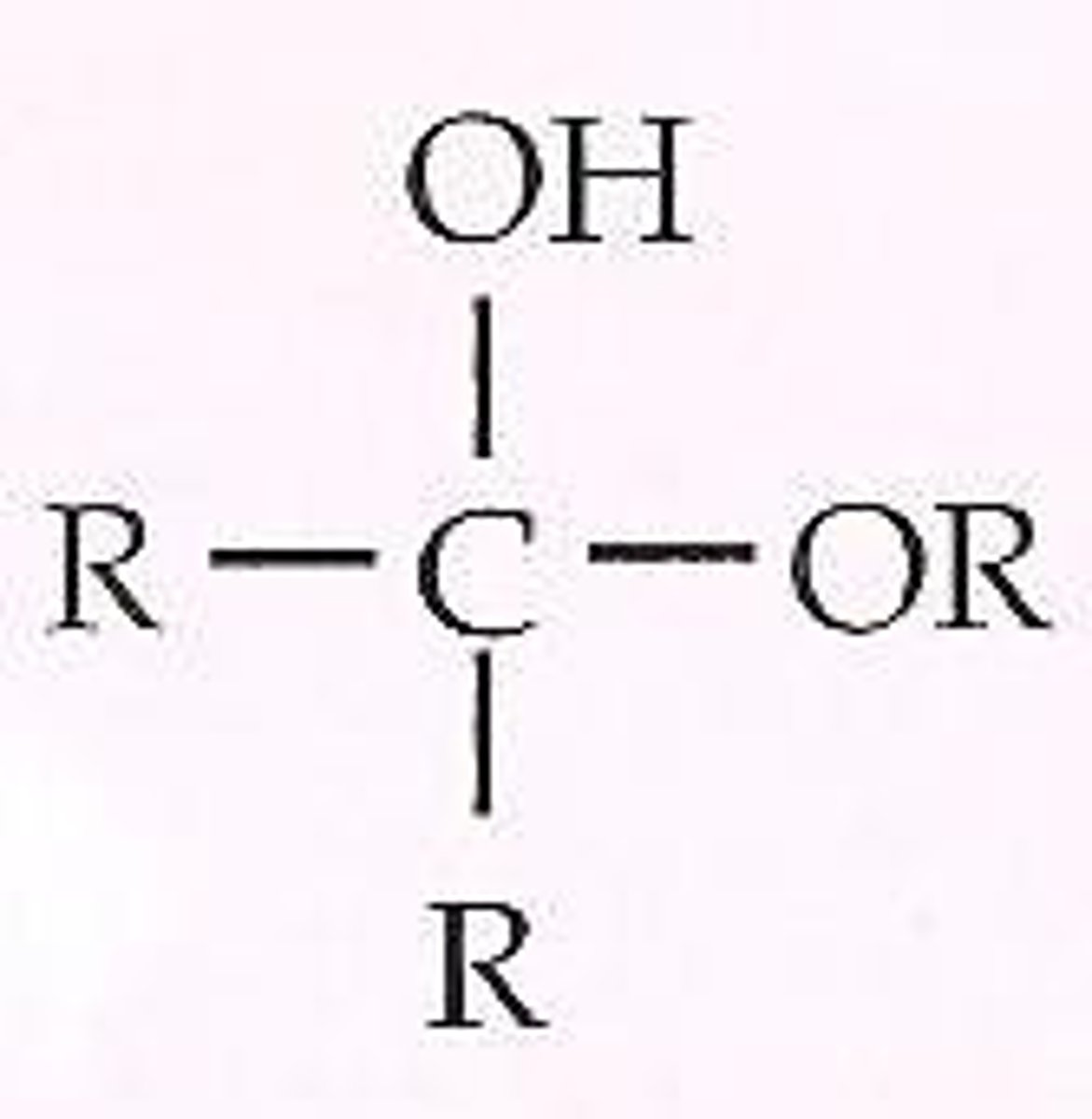
hemiketal
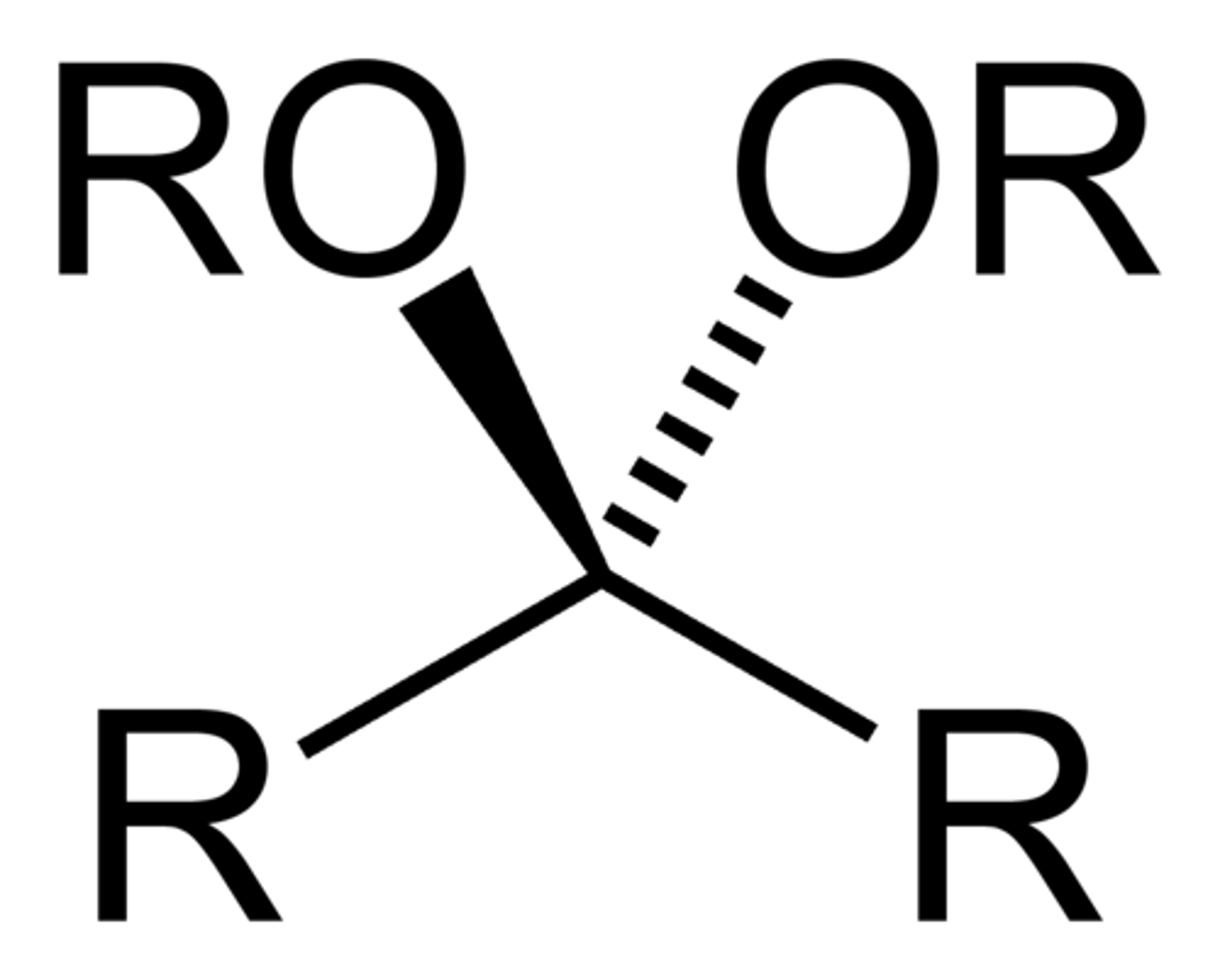
ketal
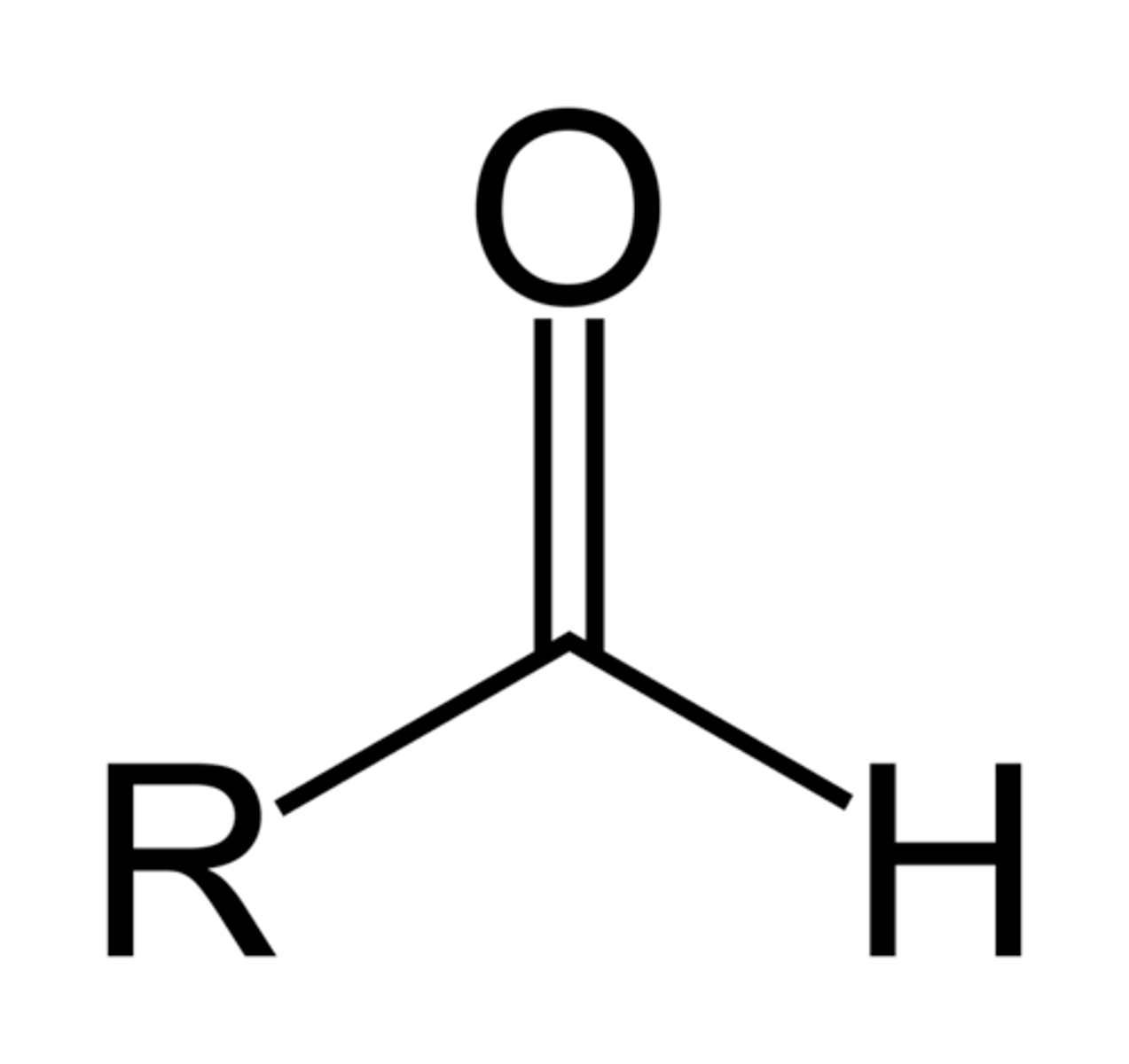
aldehyde
hemiacetal
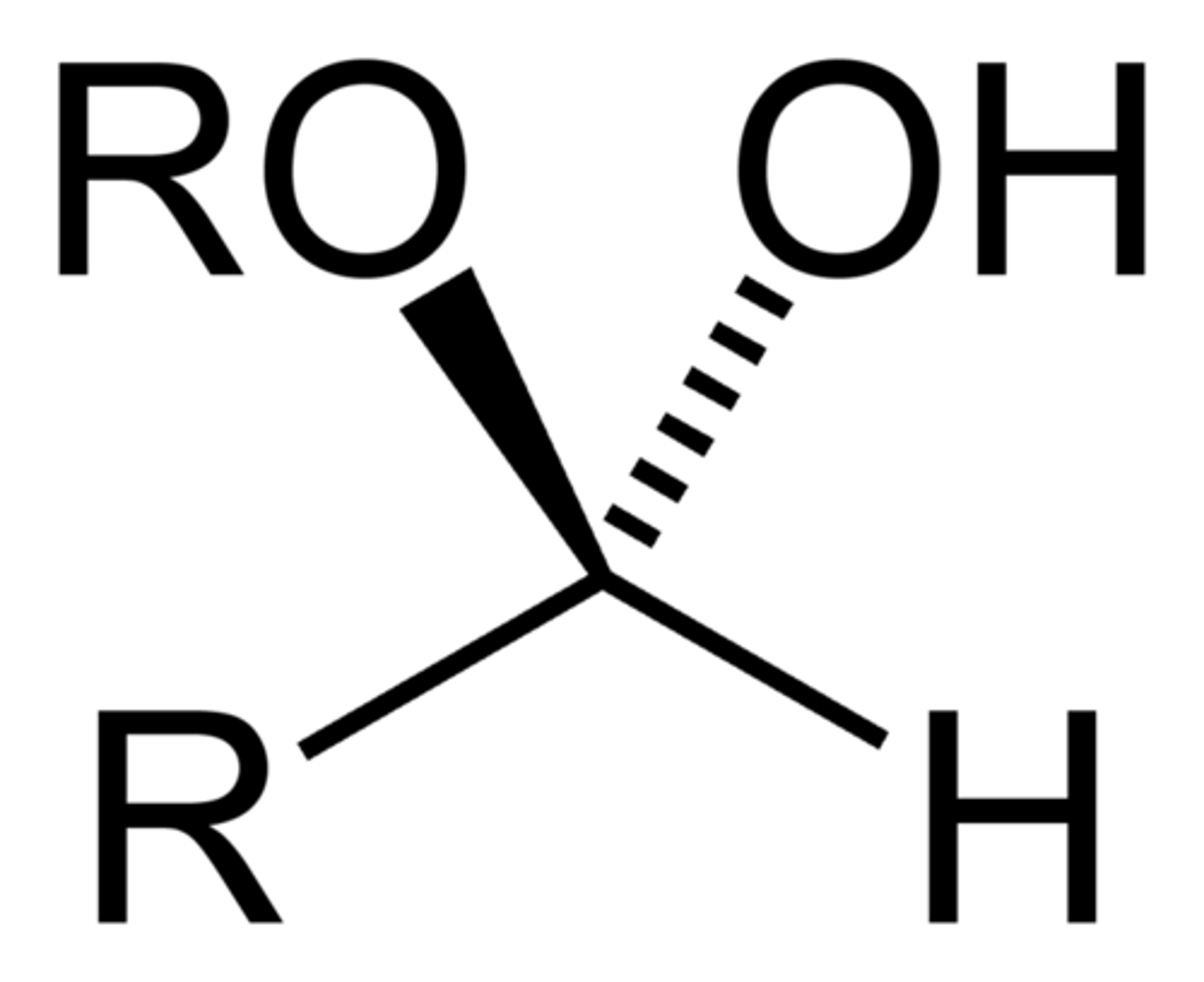
acetal
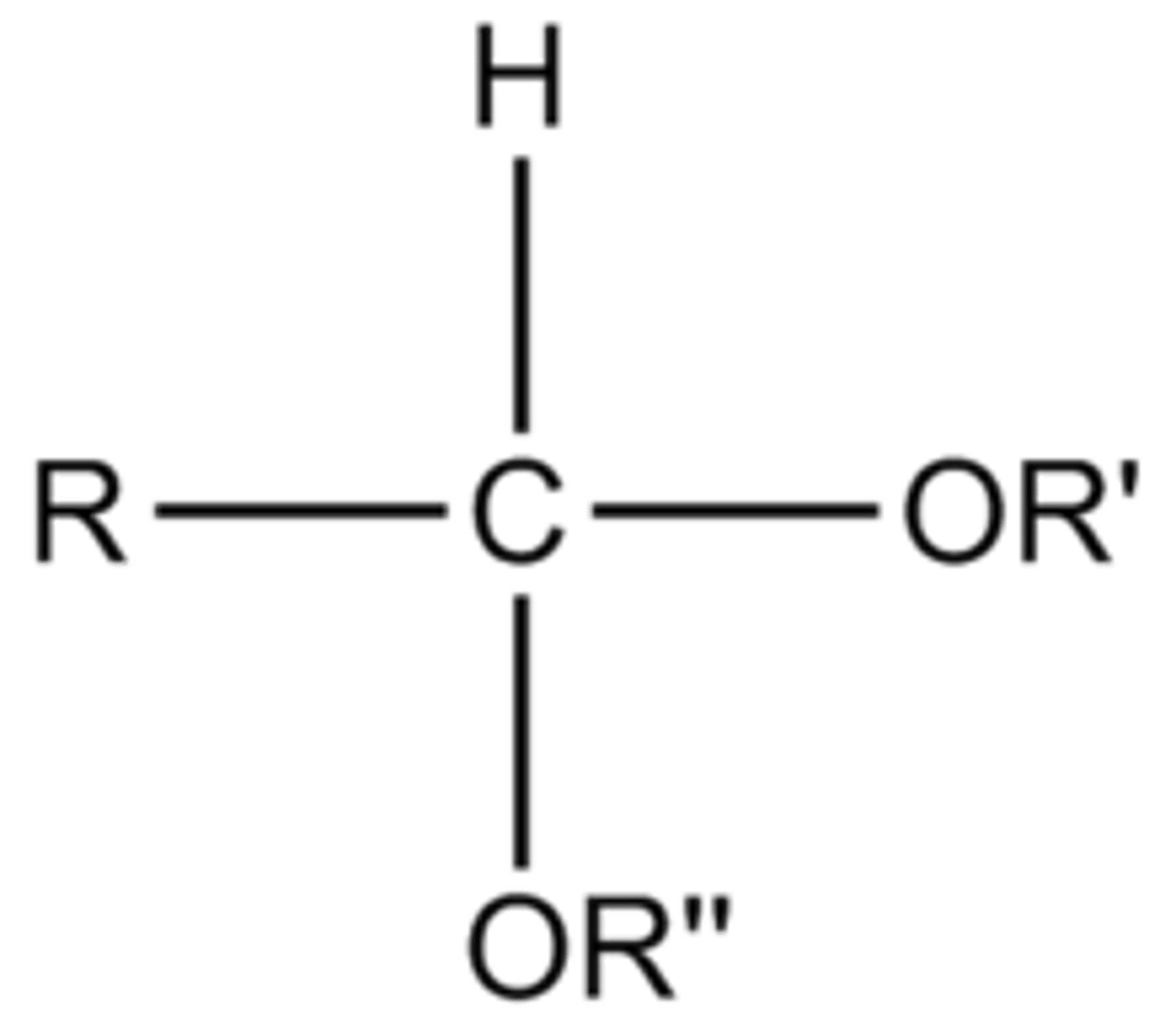
Ketals/Acetals
need strong acid to make or go back
make ketal/acetal mechanism
1. protonate oxygen in c=o
2. new thing add
3. deprotonate new thing
4. protonate leaving group (probs oh)
5. assisted ionization
6. add new OR group
7. deprotonate
8. ketal or acetal!
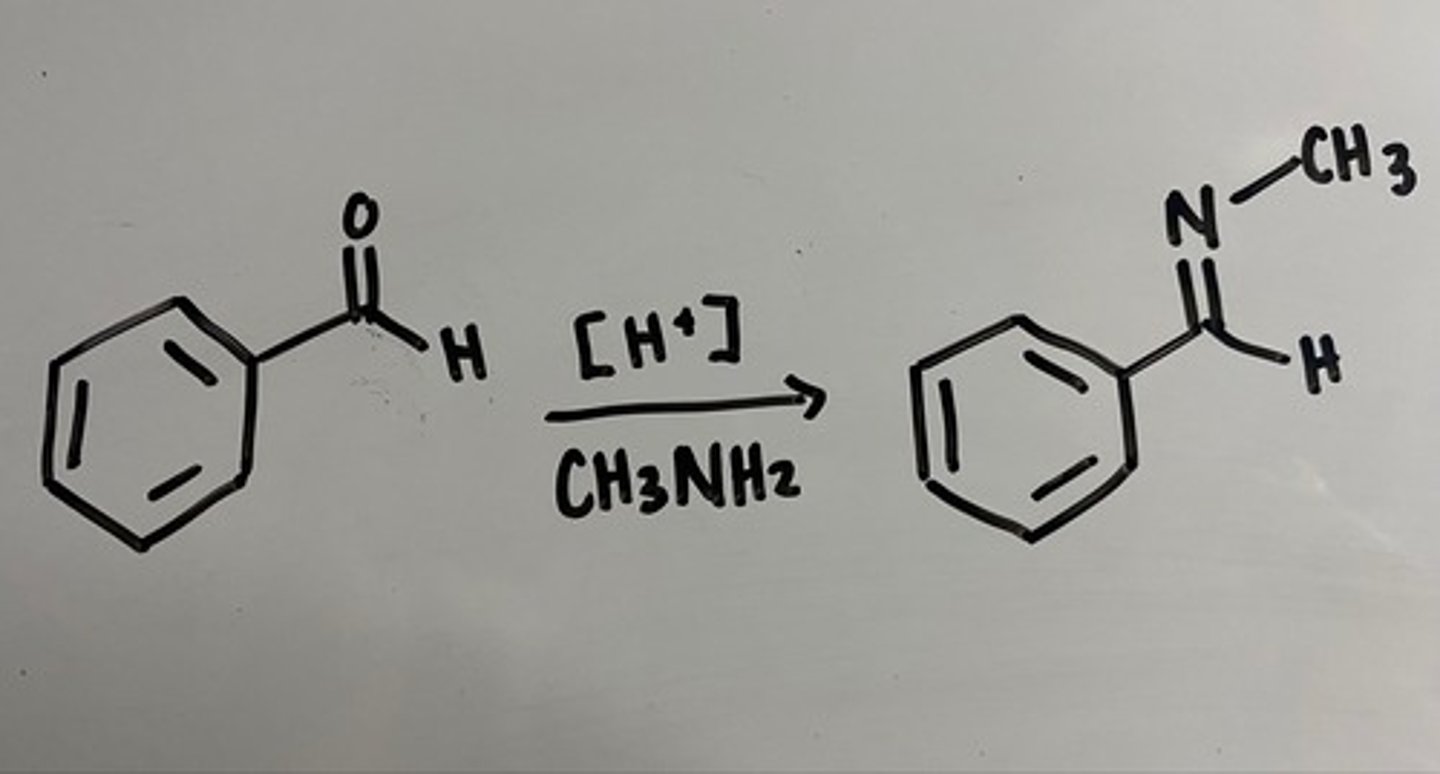
making imines
ph=6
epoxidation
mCPBA
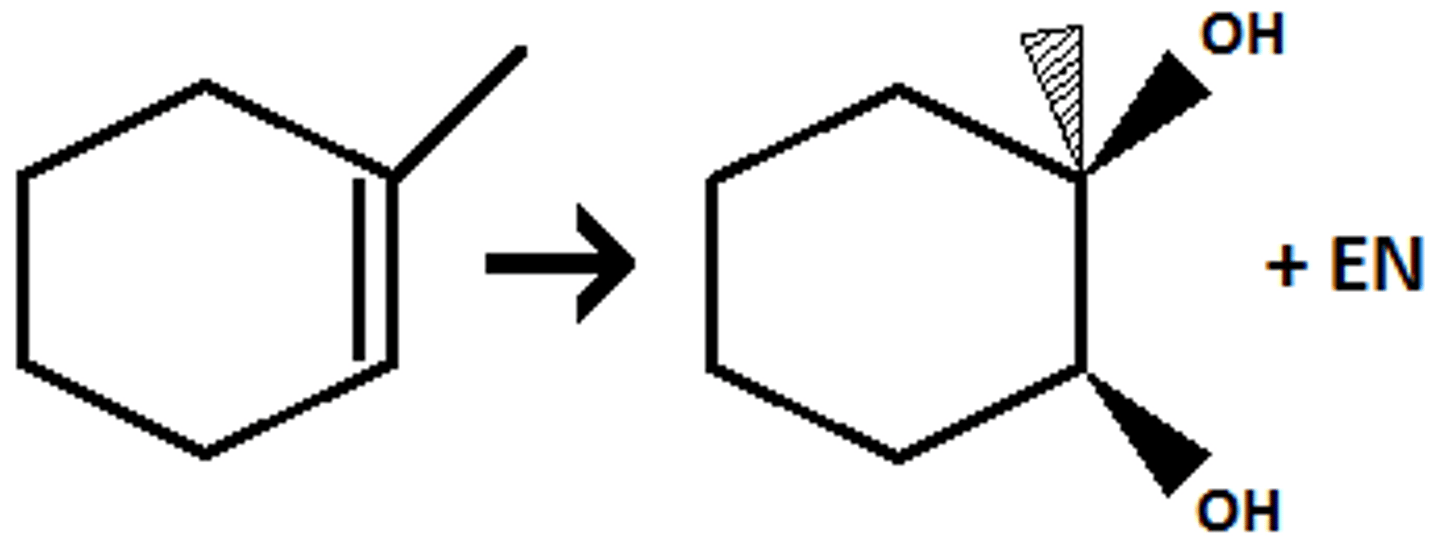
dihydroxylation
oso4, kmno4
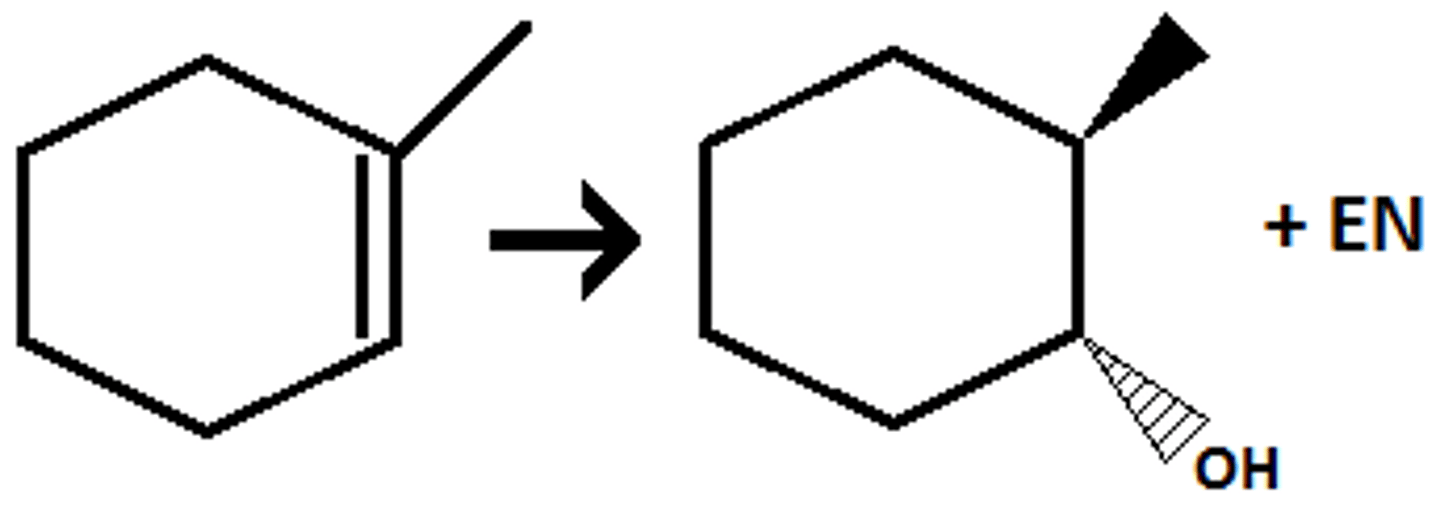
hydroboration
bh3, h202, naoh
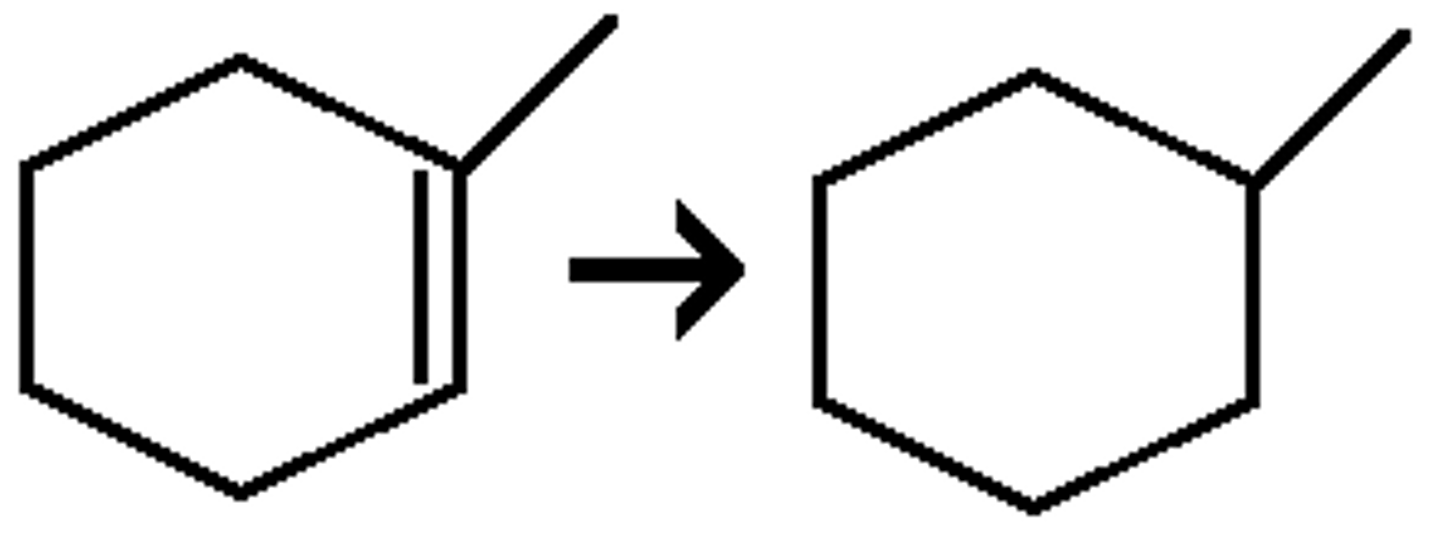
hydrogenation
triple bond to single, h2 & pd-c
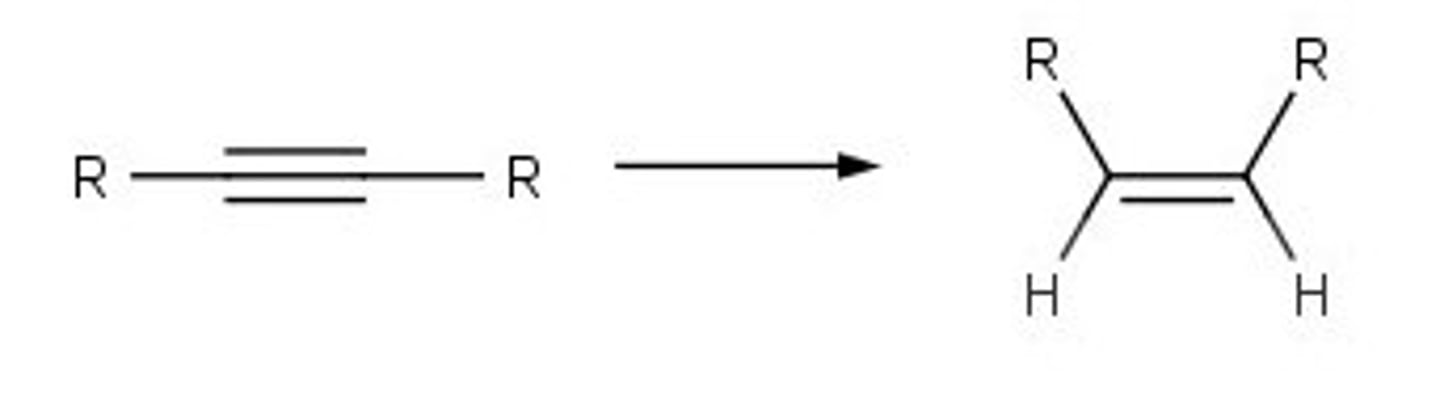
poison catalyst
triple bond to double with z double bond, h2, caco3 or pbo
dissolving metal
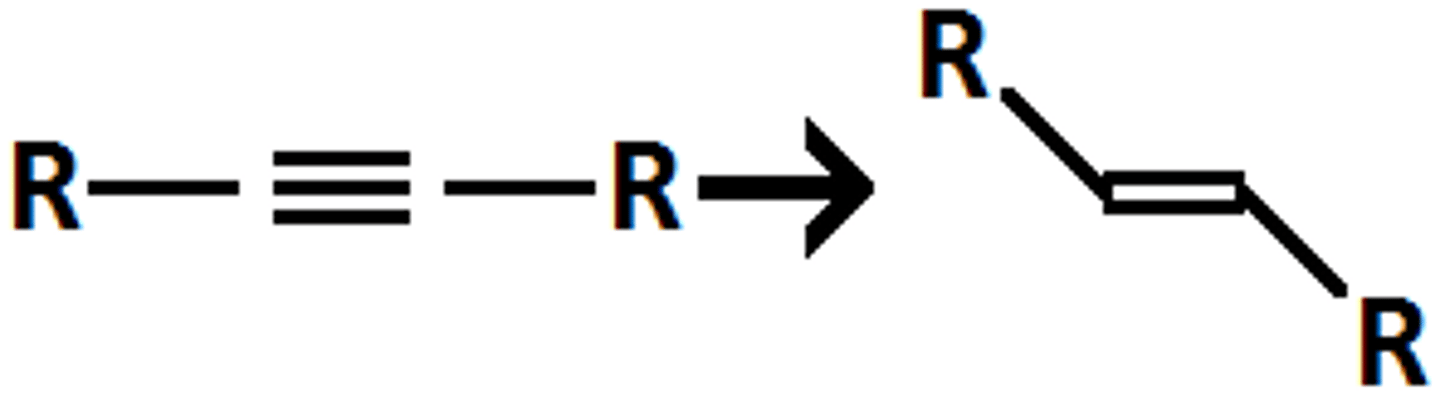
triple to double with e stereo, nanh3
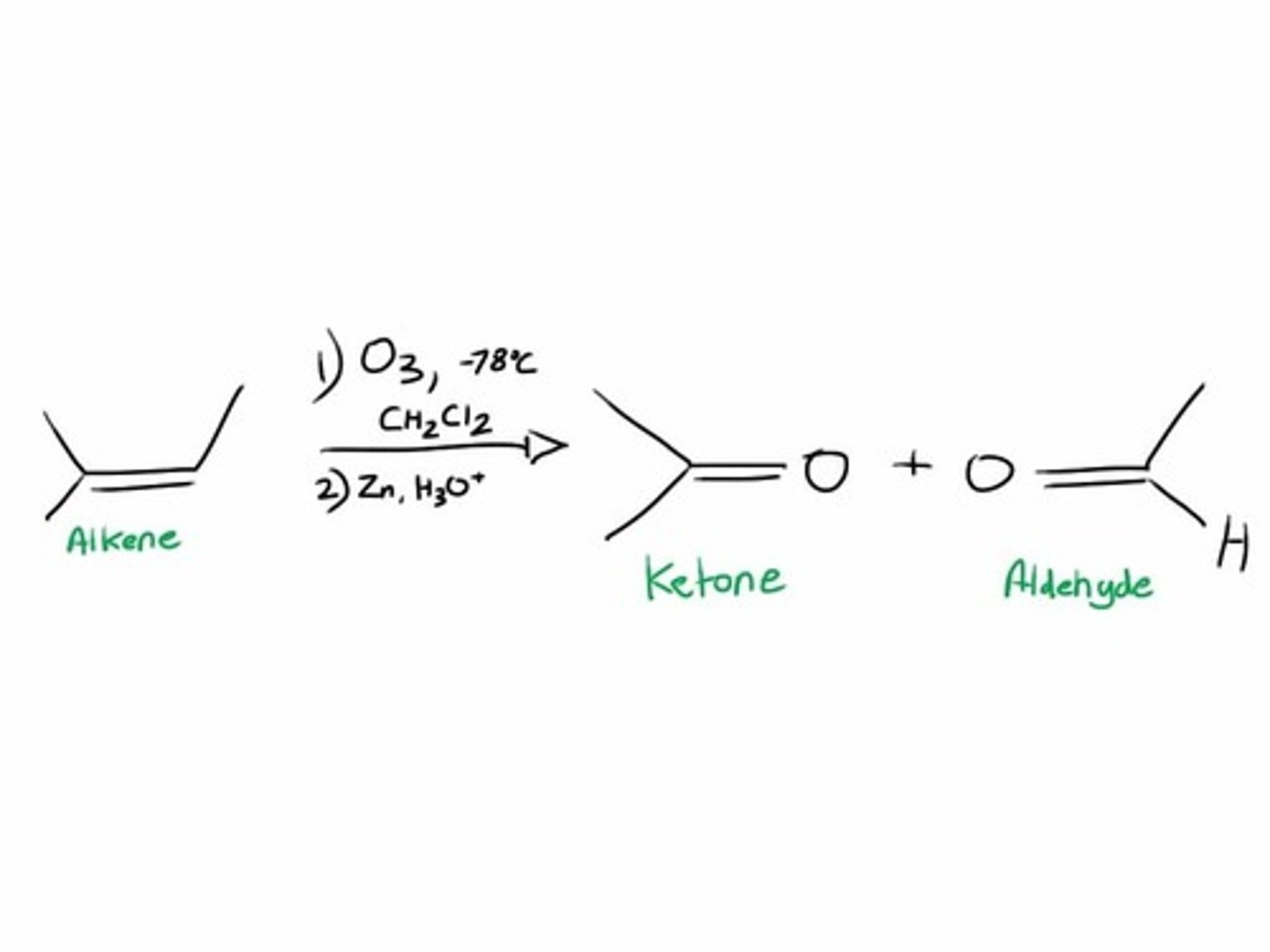
ozonolysis reductive
1. o3 2. zn
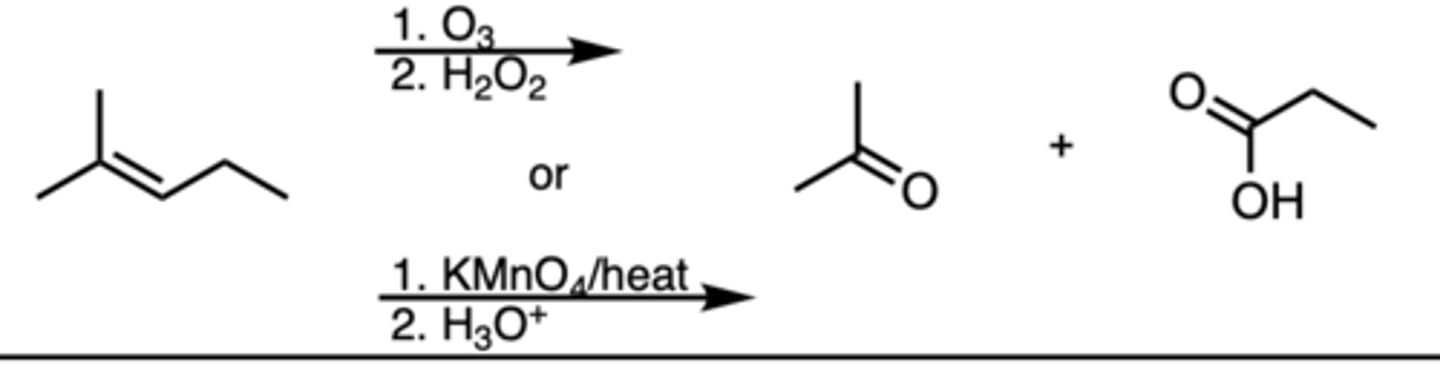
ozonolysis oxidative
1. o3 2. h202, naoh
Acid Chloride to Ester- Acylation- anionic nuc (strong base)
2 steps, NaOCH3
Acid Chloride to Ester- Acylation- Neutral Nuc + Catalyzed strong acid
6 steps, CH3OH + H2SO4, protonate carbonyl, neutral nuc attack, nuc deprotonated, protonate LG, assisted ionization of LG, deprotonate carbonyl
Acid Chloride to Ester- Acylation- Neutral nuc + weak base
3 or 5 steps, only works when x= good lg (cl, anhydride) and Nu-H is a strong nucleophile (amine). nuc attacks, nuc deprotonated, LG leaves
Acid Chloride to Mixed Anhydride- Acylation- anionic nuc (strong base)
2 steps, Na o-c=o-ph
Acid Chloride to Mixed Anhydride- Acylation- Neutral Nuc + Catalyzed strong acid
6 steps, Na o-c=o-ph + H2SO4, protonate carbonyl, neutral nuc attack, nuc deprotonated, protonate LG, assisted ionization of LG, deprotonate carbonyl
Acid Chloride to Mixed Anhydride- Acylation- Neutral nuc + weak base
3 or 5 steps, only works when x= good lg/reactive electrophile (cl, anhydride) and Nu-H is a strong nucleophile (amine). nuc attacks, nuc deprotonated, LG leaves
Amides can only go directly to
esters and carboxylic acids
Amide to carboxylic acid (-oh) works in
basic (1. NaOH, 2. H3O+) and acidic (H20 + H2SO4) conditions
Amide to ester- Acylation- anionic nucleophile (strong base)
not reversible w nucleophile, basically only strong acid works
Amide to ester- Acylation- neutral nucleophile and strong acid (1 equiv h2so4)
1. protonate carbonyl 2. nuc attack 3. nuc deprotonated 4. amide protonated 3. assisted ionization 4. carbonyl deprotonated by amide. (once amide protonated w positive charge, no longer accsessible as nuc)
esters and thioesters cant go back to
chlorides or anhydrides
Transesterification
ester to ester (only strong acid or strong base)
esters and thioesters- Transesterification- acylation- strong base
2 step, need excess of na och3 or other ester to drive rxn forward
esters and thioesters- Transesterification- acylation- neutral nuc (ROH) and strong acid (H2SO4)
intramolecular transesterification (favored). 6 steps. PROTONATE BEFORE LEAVING
esters and thioesters- Transesterification- acylation- neutral nuc + weak base doesn't work
HOCH2CH3 + triethylamine (DOESNT WORK)
Hydrolysis of esters (ester to carboxylic acid)
only strong base and strong acid work
esters and thioesters- ester to -oh - acylation- strong base
2 steps, needs h30+ work up step bc base will deprotonate oh group on carboxylic acid group
esters and thioesters- ester to -oh - acylation- neutral nuc and strong acid (H2SO4)
large excess of water (neutral nuc) drives rxn forward , 6 steps
esters and thioesters- ester to amide - acylation- strong base
DOESNT WORK
esters and thioesters- ester to amide - acylation- neutral nuc and strong acid
DOESNT WORK
esters and thioesters- ester to amide - acylation- neutral nuc and weak base (amide and triethylamine)
h-amide + triethylamine
Acyl Transfer Reactions- ester to carboxylic acid
use h20 + h2so4
acid chloride to carboxylic acid
use weak base conditions, 1. water/triethylamine 2. h30+
Reactions of RLi, RMgX w CO2
brings to carboxylic acid after h30+ workup
cant go from carboxylic acid to amide
must go oh to cl to amide
NaBH4 only works w
Acid Chloride, w workup brings to primary alcohol
Acid Chloride & LiAlH4
brings to primary alcohol with workup
Acid Chloride with MgBr-CH3
Reacts twice to bring to primary alcohol with workup
Acid Chloride with Ch3-Li (excess)
Reacts twice to bring to primary alcohol with workup
Esters (thioesters too) with LiAlH4 (excess)
Reacts twice to bring to primary alcohol with workup
Esters (thioesters too) with Ch3-MgBr (excess)
Reacts twice to bring to primary alcohol with workup
Esters (thioesters too) with Li-Ch3 (excess)
Reacts twice to bring to primary alcohol with workup
Esters (thioesters too) with NaBH4 (excess)
no reaction
Amides only react with
LiAlH4, swaps out carbonyl for two h's
Carboxylic Acids w NaBH4
no reaction
Amides with Mg-X or Li-X
no reaction
Carboxylic Acid with LiAlH4
Reacts twice to bring to primary alcohol with workup
Carboxylic Acid with CH3-Li
Reacts once, carbonyl maintained
Carboxylic Acid with Ch3-MgBr
no reaction
Weinreb Amide with NaBH4
no reaction
Weinreb Amide with LiAlH4
turns to an aldehyde (weinreb amide leaves)
Weinreb Amide with CH3-Li
turns to a ketone (weinreb amide leaves) (gets stuck at tetrahedral intermediate)
Weinreb Amide with CH3-MgBr
turns to a ketone (weinreb amide leaves)
Claisen
enolate +ester
Aldol addition
enolate attack aldehyde or ketone leaving alcohol bc nothing can be kicked off
Aldol Condensation
same as aldol addition, except enolate formed again to kick off alcohol leaving c=c bond
Decarboxylation (ketone and ester)
H2so4 and h20 to turn ester into carboxylic acid, heat to creat CO2 (heat yeets co2 out)
enols (strong acid)
no sn2
LiAlH4, NaBH4
1, 2 addition
Ch3Li, MgBr-CH3
1, 2 addition
Ch3Li, MgBr-CH3 with CuI
1, 4 addition
Heteroatoms
1, 4 addition