Unit 1: Biochemistry - #5 Structure and Function of Macromolecules: Proteins
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
Proteins
Make up 50% of the dry mass of most cells
Structural value: Collagen (tendons, bones) and keratin(hair, nails)
Enzymes that are used as catalysts in chemical reactions
Transport materials throughout the body like oxygen and carbon dioxide
Produce antibodies that destroy foreign bacteria and viruses
Form structures that allow transport across the membrane
Structure
Proteins are large polymer units made up of amino acids monomers
The overall shape of a protein is determined by the amino acids that it is composed from
The structure of a protein is important in determining its overall function. It has to be the EXACT fit because if the shape changes, then the protein may not be able to perform its function properly
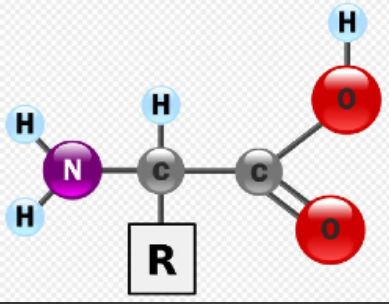
Structure of Amino Acids
Consist of a central carbon bonded to four different covalent partners
Hydrogen atom
Carboxyl
Amino group
R group
There are 20 different R groups which results in 20 different amino acids
R groups give amino acids their properties (polar, non-polar, acidic, etc)
8 amino acids are considered essential - only obtained through diet
Amino Acid Linkages
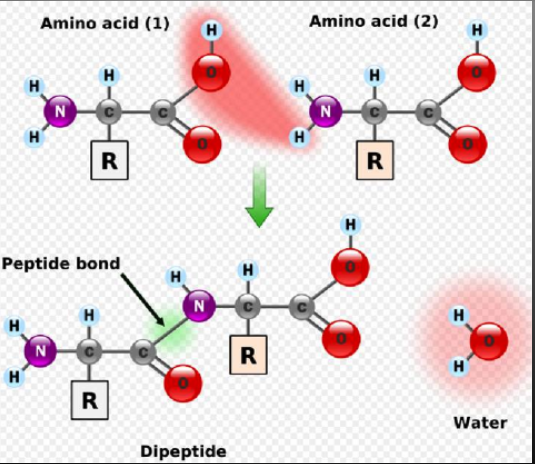
Amino acids are linked together through condensation (dehydration synthesis) reactions
The carboxyl group of one amino acid bonds to the amino group of a second amino acid to form a peptide bond.
When two amino acids bond, the resulting molecule is called a diepeptide.
The chain of more than 50 aa’s(amino acids) is called polypeptide
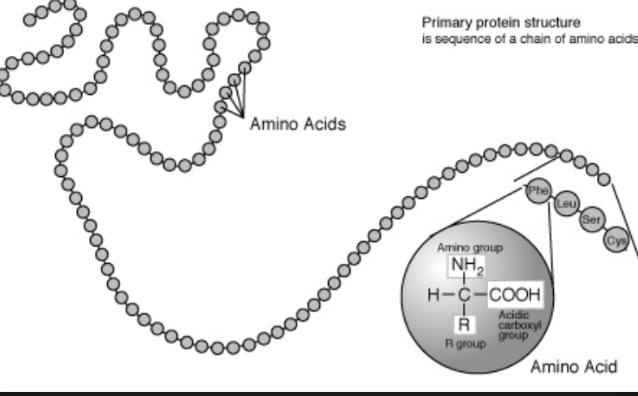
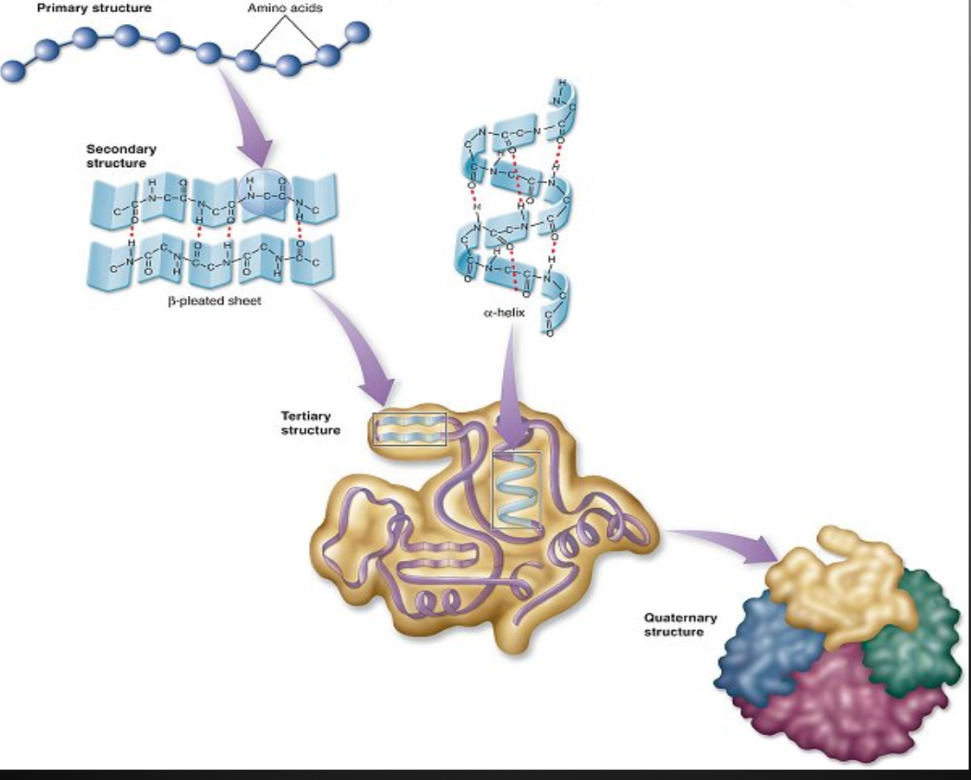
Primary Structure
Unique linear sequence of amino acids in a polypeptide chain
Changing a single amino acid will alter the overall structure of the protein
Unlimited combos of primary structure, specific to each protein (20 combos for each spot of the chain)
Secondary Structure
Results from hydrogen bonding between carboxyl group of one amino acid and the amino group of a neighboring amino acid
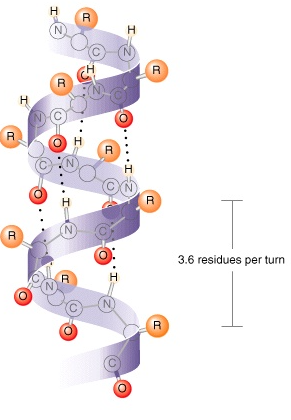
𝛂-helix
Coil structure held together by h-bonds between every fourth amino acids
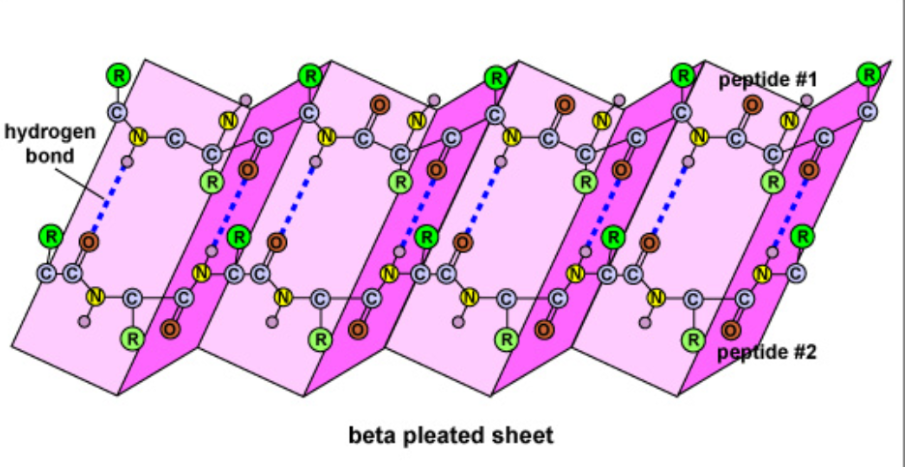
ꞵ-pleated sheet
Two separate polypeptide strands that run parallel to each other interact due to H bonds, an accordion shape appears
Ex. Strength of silk
Tertiary Structure
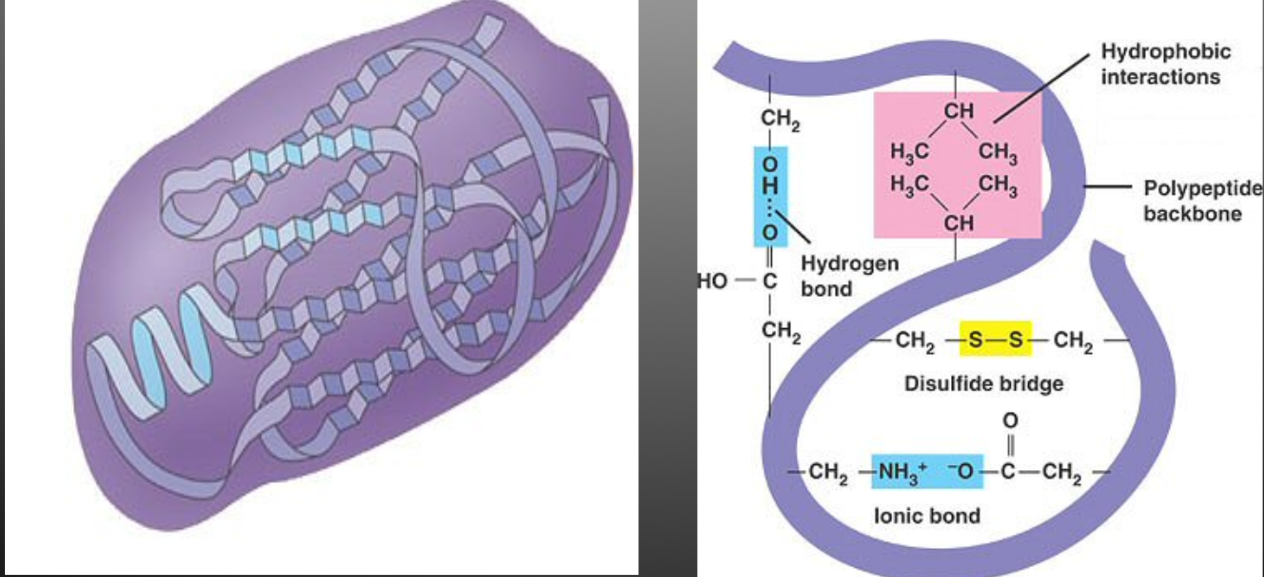
The polypeptide chain continues to bend, fold and contort itself as a result of the interaction between the “R” groups.
Polar, non-polar and ionic “R” groups interact to form hydrogen, covalent, and ionic bonds
Forms a large globular arrangement
EG. Amino acid cysteine contains a sulfur atoms that will form a disulphide bridge with another cysteine atom
Quaternary Structure
Some proteins consist of two or more polypeptide chains combined into one functional macromolecule
Same types of bonds/interactions as tertiary structure
The final structure of a protein (confirmation) is critical as its orientation and shape is directly related to its function. Many diseases and disorders are a result of an improperly functioning protein.
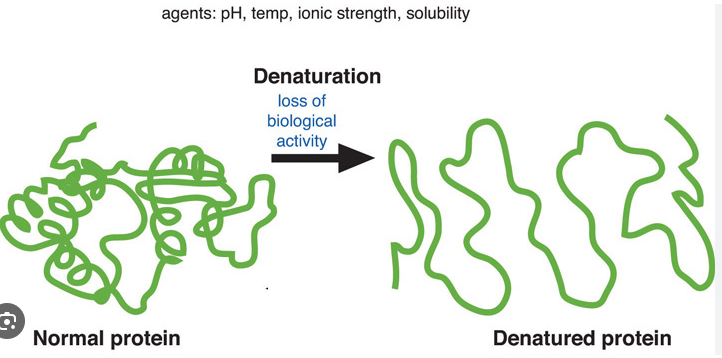
Protein Denaturation
Results from changes in the 3D shape caused by temperature, PH or ionic concentration changes
Protein unravels and looses conformation
If peptide bonds break the protein is destroyed
Enzymes function best within a narrow range of the above conditions