Protein Sorting and Post-Translational Modifications
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
26 Terms
Why do we have compartments within the cell?
establish a physical boundary
generate specific micro-environments
establish specific locations where certain processes occur
describe the process in which specific proteins have to be within specific organelles
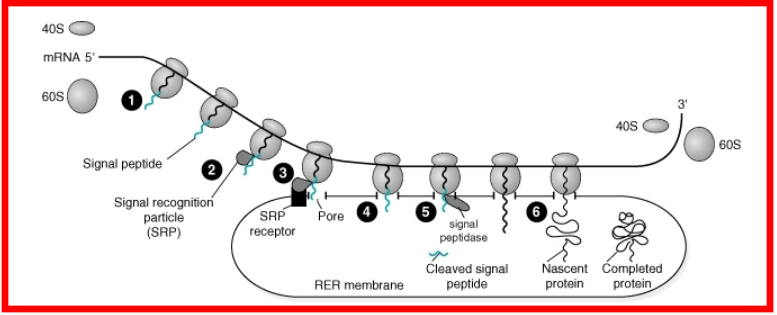
Synthesis begins in cytosol
Elongation continues on ER
Synthesized polypeptide is released inside of ER
what are the AA sequences for the mitochondria and nucleus called?
Nuclear proteins – nuclear localization signal
Mitochondrial proteins – mitochondria entry signal
What are secretory vessicles? what are some examples of molecules that needs secretory vessicles?
What are extracellular vesicles and what are their clinical importance?
Secretory vesicles contain molecules
destined for secretion outside the cell:Gastric acid
Digestive enzymes
Lung surfactants’
Sebum to lubricate skin and hair
carry proteins and nucleic acids
linked to metastatic cancers
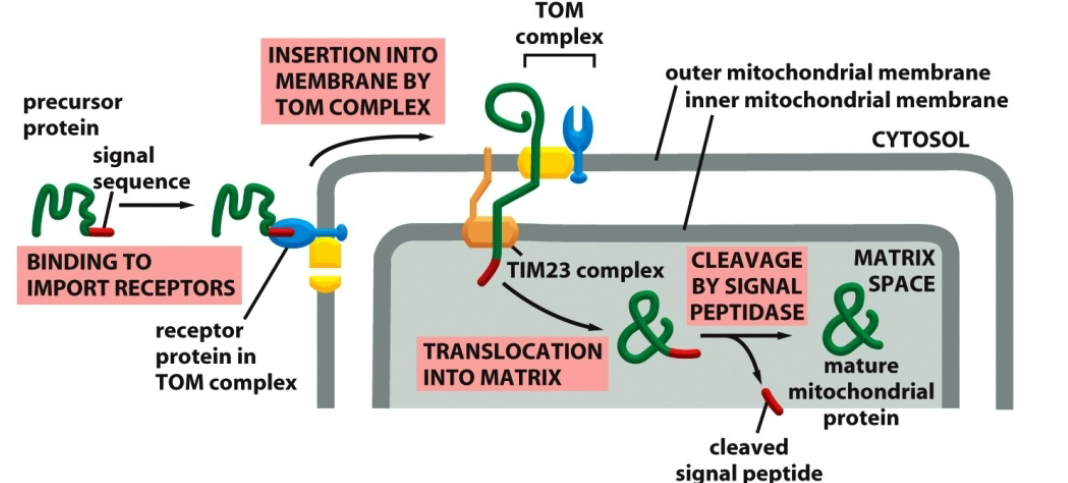
describe the process in a protein translocating to the mitochondria.
A precursor of mitochondrial protein contains a signal sequence that binds to the receptor on mitochondrial outer membrane.
With the assistance of a TOM/TIM23 complexes a precursor translocates into mitochondrial matrix.
The signal sequence is cleaved producing a mature protein.
list out the covalent modifications and where they attach to.
Phosphorylation and dephosphorylation: Ser, Thr, and Tyr.
Ubiquitination: Lys
Acetylation: Lys
Methylation: Lys and Arg
Hydroxylation: Pro and Lys
Carboxylation – blood coagulation (glutamate)
Attachment of fatty acids to anchor protein in membrane.
Glycosylation: Ser, Thr, or Asn
Glycation: Arg or Lys
List out some protein kinases.
tyrosine kinases, serine-threonine kinases
What is Chronic myelogenous leukaemia? Describe its pathology.
cancer of bone marrow → too many white blood cells.
Fusion of ABL1 (chromosome 9) gene with BCR gene (chromosome 22) = BCR-ABL1 (aka, philadelphia) —> protooncogene tyrosine protein kinase.
This protein phosphorylates multiple targets in leukemia cells → uncontrolled proliferation
where does ubiquitin attach? What are the two types and their functions? What are the official names of E1, E2, E3?
amino group of lysine
Monoubiquitination: DNA repair, regulation of transcription and internalization of transmembrane proteins.
Polyubiquitination is involved in proteosomal degradation of modified protein
ubiquitin-activating (E1),
ubiquitin-conjugating (Ubc),
ubiquitin ligase (E3)
what is the VHL gene and what happens if it is defective?
Encodes E3 ubiquitin ligase; target hypoxia-inducible transcription family factor (HIF) for destruction.
Mutations in VHL gene impair its function and
promote angiogenesis and renal tumor.
How is BRCA1 responsible for breast cancer?
human tumor suppressor gene and is responsible for DNA repair.
It has E3 ubiquitin ligase activity.
Mutation that affects the ubiquitin ligase function are
found in various cancers.
Which Amino Acid does acetylation occur? Give examples of acetylation
most common = lysin; at n-terminum
Examples:
Acetylation of COX1/COX2 by aspirin.
Histone acetylation by histone acetyltransferase (HAT)
promotes transcription
Deacetylation by histone deacetylase (HDAC) suppresses transcription.
Describe the relationship between p53 and acetylation
Acetylation of tumor suppressor p53 is necessary for
its activation.
A suppression of p53 acetylation can:
Prevent it from activation of p23
Lead to the loss of cell growth control.
Prevent p53 from performing its proapoptotic functions.
Increases the incidence of cancer
Which Amino Acids does methylation attach to? Which enzyme? What are some of the effects of methylation?
catalyzed by methyltransferases and
demethylases.Addition usually occurs on the nitrogen side-chain of lysine or arginine.
Some functions:
Regulation of transcription
Modulation of enzymatic activity
Protein trafficking
Signal transduction
What two enzymes are active in methylation of histones? How does this affect transcription? Give two examples
histone methyltransferase
SAM (methyl donor)
Affects transcription by activation or repression
Trimethylation of histone H3 at
Lys 4 (H3K4me3) is an active
mark for transcription.Dimethylation of histone H3 at
Lys 9 (H3K9me2), a signal for
transcriptional silencing.
Which AA does hydroxylation take place? How does this relate to collagen? Describe this hydroxylation reaction
at proline
stabilizes the secondary structure of collagen due to the strong electronegative effects of oxygen
catalyzed by a multi-subunit enzyme: prolyl 4-hydroxylase.
requires iron and α-ketoglutarate for oxidation and to return iron to its oxidized state
Describe the pathology of scurvy. Symptoms?
cause by vitamin C deficiency
Collagen needs Vitamin C for its stability as vitamin C return the iron used in prolyl 4-hydroxylase to its oxidized state;
lacking thereof results in:
Decreased red blood cells
Gum disease
Poor wound healing
What is carboxylation? Where does it occur? How is it catalyzed? What is its cofactor?
posttranslational modification: glutamate (Glu) → γ carboxyglutamate,
Carboxylation occurs in the liver and is performed by γ-glutamyl carboxylase.
Requires vitamin K as a cofactor.
What types of protein does carboxylation primarilly affect? Describe these proteins’ functions
carboxylation primarily affect proteins in the blood clotting cascade: specifically factors II, VII, IX, and X
Function:
γ-carboxyglutamate binds calcium, which is essential for its function.
In prothrombin (factor II):
calcium binding —> protein association with plasma membrane of platelets
Bringing it closer to protein that cleaves prothrombin to thrombin
Describe how GPI-linked proteins are produced? What are some examples of this protein?
Glycosylphosphatidylinositol-anchored (GPI-linked) protein
produced in the ER
How it is produced:
Protein co-translationally inserted into ER membrane.
hydrophobic C-terminus cleaved off and replaced with GPI-anchor by
GPI-transamidase.Then, translocated to Golgi and then to the plasma membrane.
Examples:
Antigens; human carcinoembryonic antigen (CEA) is used as a cancer marker.
Enzymes; alkaline phosphatases and acetylcholinesterase
Describe the relationship between GPI-anchored proteins and RBC
GPI-anchored proteins on the surface of RBCs protect them from destruction by the complement system.
What is PNH? What causes it? consequences?
paroxysmal nocturnal hemoglobinuria
somatic mutation in X-chromosomal gene PIGA
this is an important component for GPI-transamidase.
Consequences:
destruction of RBC by compliment system
50% of individuals die through thrombotic complications
increased risk of leukemia
What is glycoslyation? Which types of proteins are modified this way? what is glycosylation important for?
covalent attachment of carbohydrate to the
target molecule.
Most soluble and membrane-bound proteins expressed in the endoplasmic reticulum are glycosylated:
Secreted protein
Surface receptors
Glycosylation is important for:
Protein folding
Cell-cell adhesion
ABO blood group
Glycosylation is used by viruses to shield from immune recognition
Compare and contrast N and O glycosylation. What determines whetehr a protein has N or O glycosylation?
N: Glycan bind to the amino group of Asn in the ER.
O: Monosaccharides bind to the hydroxyl group of
Ser or Thr in the ER, Golgi, cytosol and nucleus
Type depends on
enzyme availability
Amino acid sequence: Asn for N-linked or Ser/Thr for O- linked.
Occurs in ER and Golgi
What is glycation? Describe this reaction.
non-enzymatic covalent attachment of carbohydrate
to Arg or Lys of the target molecule.
Reactions are:
very slow and occur mainly in the bloodstream.
They result in the formation of advanced glycation endproducts (AGEs). AGEs form in hyperglycemic conditions and/or the natural process of aging.
What is the clinical importance of AGEs? How does diet impact presence of AGEs
AGEs implicated in:
Deafness
Alzheimer’
Cardiovascular
Cancers
AGEs causes altercations to these organs:
Heart and vasculature – vessel stiffness
Vitreous body – affect vision
Skin – skin stiffness
Lung collagen – affect respiration
Intervertebral discs – decreases flexibility
We measure AGEs via hbA1c for diabetes
A significant generation of AGEs occurs when sugars are cooked with proteins.
It has been reported that, in renal failure patients, there was a 29% increase in glycation levels in the blood of those subjected to an AGE-rich diet