Engineering materials
1/56
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
57 Terms
(Classification) Engineering Materials are generally classified into which 4 groups
metals, polymers and elastomers, ceramics, composites
(Classification) Materials can be classified according to their
- properties
- the way they occur in nature
- the way they are prepared
- atomic/crystal structure
- industrial application
(Classification) Metals general properties
- lustrous (when oxide layer is removed)
- usually solid at room temp
- usually malleable and ductile
- usually good conductors of heat and electricity
- able to form alloys
(Classification) Non-metals general properties
- usually dull if solid
- all room temp gases are non-metals
- usually brittle if solid
- usually insulators of heat and electricity
- unable to form alloys but can form compounds
(Mechanical Properties) Strength
the ability of a material to withstand applied loads without failure, and varies according to the type of load applied (i.e load, tensile, compressive, shear, torsional)
(Mechanical Properties) Hardness
the ability of a material to resist scratching, abrasing or indentation
(Mechanical Properties) Elasticity
the ability of a material to return to its original shape and dimensions after being subjected to a load
(Mechanical Properties) Toughness
the ability of a material to absorb energy up to fracture
(Mechanical Properties) Stiffness
the ability of a material to resist elastic deformation under load (measured using Young's modulus)
Stiffness vs Elasticity
Elasticity describes a material's ability to return to its original shape after deformation, whereas stiffness describes how much force is required to deform a material or structure.
(Mechanical Properties) Plasticity
the ability of a material to undergo some degree of permanent deformation without rupture. (opposite of brittleness) This quality increases at higher temperatures, therefore rolling, extruding, pressing, forging, and spinning are done at elevated temps.
(Mechanical Properties) Malleability
the ability of a material to be hammered and rolled into thin sheets
(Mechanical Properties) Ductility
the ability of a material to be drawn out into thin wire. (% elongation in tension test) Not all ductile materials are malleable
(Mechanical Properties) Fatigue
the tendency of a material to break when subjected to repeated cyclic loading where the induced stress is well below the elastic limit
(Mechanical Properties) Notch Toughness
a measure of the amount of energy required to cause failure
(Physical Properties) Density
the amount of matter packed into a given volume.
p (density kg/m^3) = m (mass kg) / V (volume m^3)
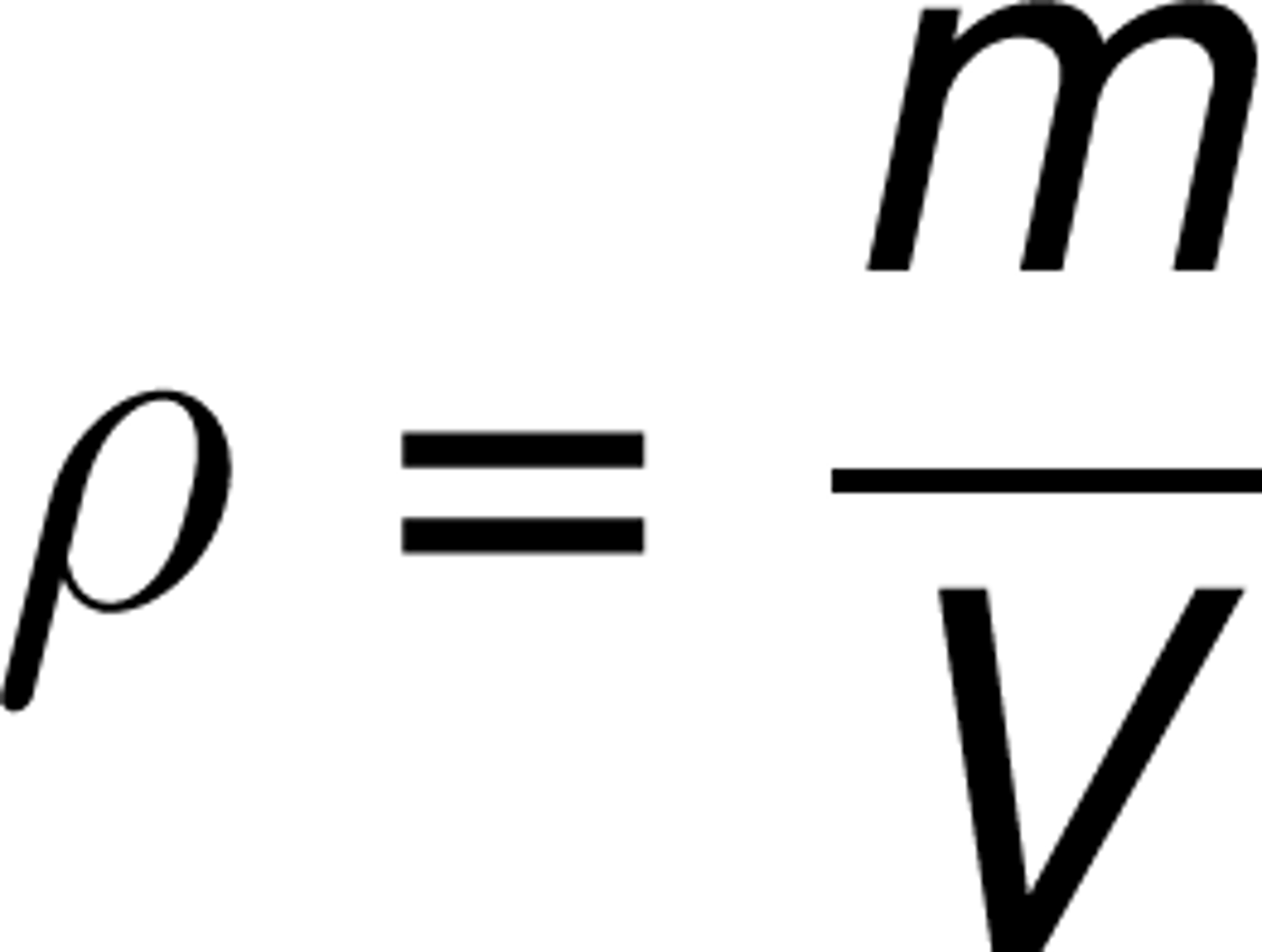
(Physical Properties) Porosity
a measure of the amount of voids or pores a material has. May naturally occur (timber) or be introduced in manufacture
(Physical Properties) Moisture Content
a measure of the amount of moisture present in the structure of a material. Is great importance to timber, high moisture content reducing strength, and increasing thermal and electrical properties. Some polymers can also absorb moisture, which has an impact on strength and electrical properties
Ionic bond
Metal + Non-metal, electrons traded
Covalent bond
Non-metal + Non-metal, electrons shared
Metallic bond
- Metals have 1-3 e-, valence shell is far from nucleus
- Valence electrons condense to form a 'sea' of electrons
- Atoms become positive ions and repel each other
- Stabilised by the ions' attraction to the electron cloud
- Equilibrium causes the ions to form a regular (crystalline) pattern
Advantages of metallic bonds in metals
- free electron cloud allows for electrical conductivity
- free electrons repel photons, making metals 'shiny' (opaque)
- allows for malleability as positive ions move relative to one another
Liquids structure
Little or no order
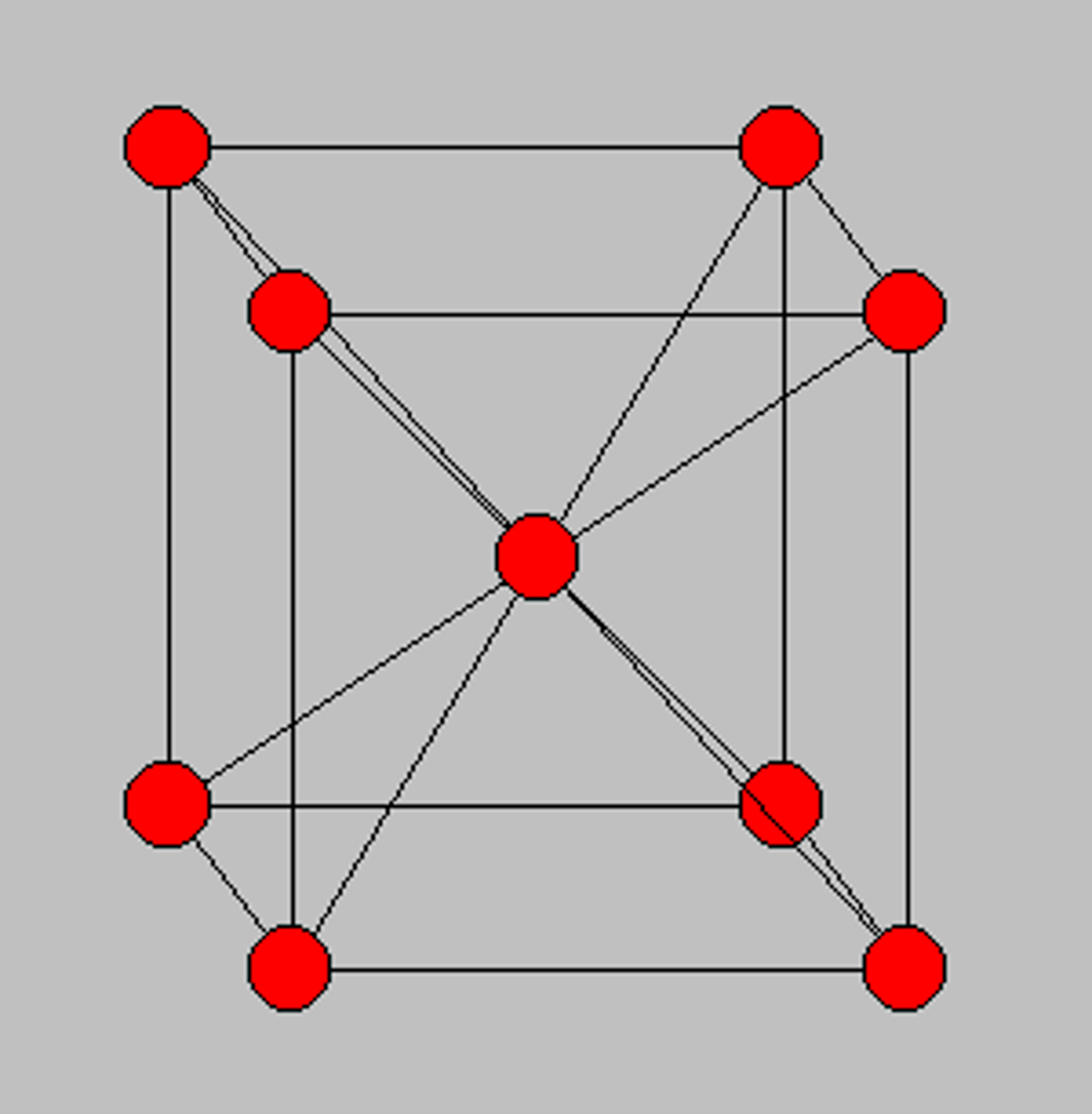
BCC
Body Centred Cubic
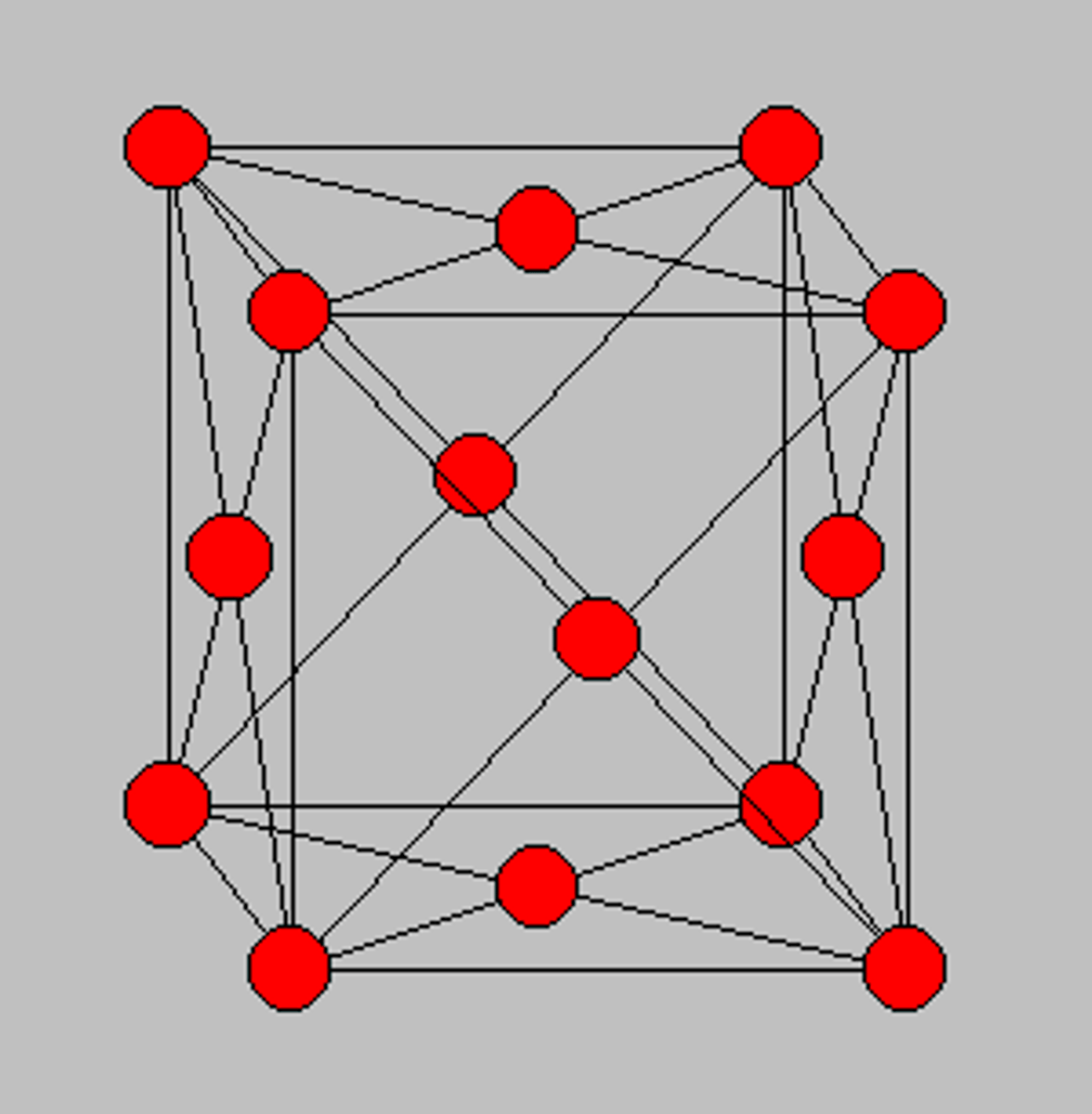
FCC
Face Centred Cubic
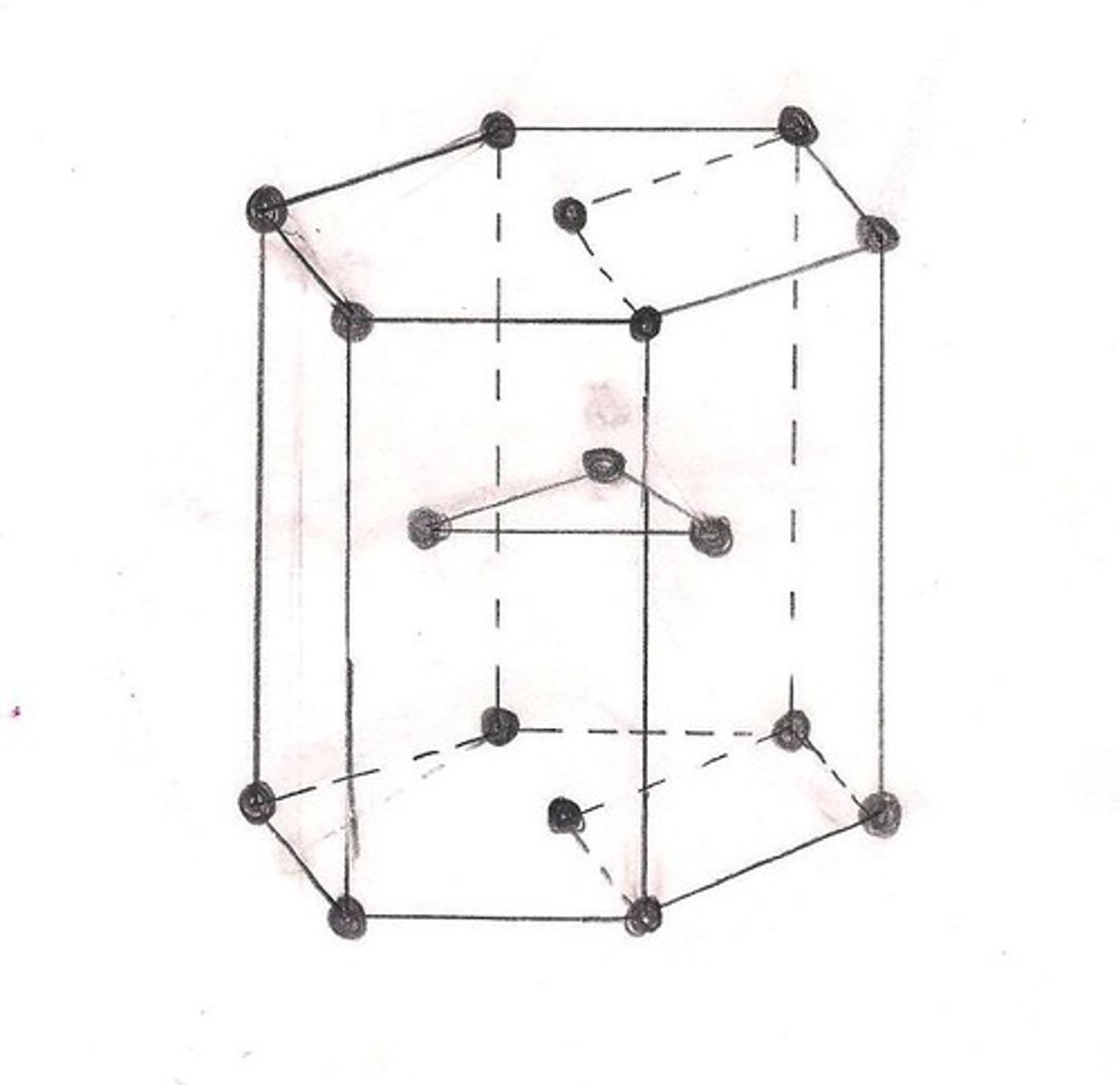
HCP
Hexagonal Close Packed
BCC features
- 2 atoms per unit (fairly open structure)
- less dense --> more interlocking and less slip
- low ductility
- e.g Cr, W, V, Na, steel at room temp.
FCC features
- 4 atoms per unit (fairly closed structure)
- more dense --> less interlocking and more slip
- high ductility
- e.g Al, Ni, Cu, Au, Pb, Ag, Pt, steel at red hot
CPH features
- similar density to FCC
- however, the way CPH's atoms are arranged results in fewer slip planes, and therefore lower ductility than FCC and BCC
- e.g Zn, Mg, Cd, Be, Ti
(Another name for) Non-crystalline materials
amorphous (when describing the structure of a solid)
Amorphous solids features and examples
- some polymers and ceramics
- glass
- often unstable
Glass
- is cooled to rigidity without crystallising
- can be described as a liquid with a very high viscosity
Polymorphism
- aka allotropy
- a metal that has more than one crystalline structure
- e.g iron, at room temp., is BCC, at red hot FCC and BCC at hotter temperatures
(The growth of a grain) liquid state
crystals form but immediately break due to energy (heat)
(The growth of a grain) solidification begins
nucleation begins by the formation of BCC FCC or CPH cells
(The growth of a grain) growth of grain
grows from original cell, dendrites spread to dissipate heat
(The growth of a grain) grain boundaries
- formed when dendrites meet
- are an area of disorder
- are 2-3 atoms wide
- are areas where impurities gather during solidification
- are as strong as the grains
- faster cooling > more dendrites needed > more grains > higher strength and hardness > lower ductility
- slower > opposite of above
Dislocation theory
Metals work harden because of small dislocations in their crystal structure, formed by different cooling rates within the metal, causing internal stresses and eventually local plastic deformation (a dislocation).
Work hardening
Dislocation movement (plastic deformation) can be interfered by:
- grain boundaries
- foreign atoms
- other dislocations
- when metal is worked, dislocations are mobilised and build up at these places, eventually distorting the lattice and failing in a brittle manner
Ferrous Metal
Are metals and alloys that have iron as the primary component, (generally more than 50% in weight)
pure iron
- very soft and has little uses
- has a BCC structure (ferrite)
steels
ferrous alloys with up to 2% carbon
cast irons
- ferrous alloys with 2-5% steel
- generally cast in a mould
ferrite
- BCC
- aka alpha (α) iron
- has up to 0.025% carbon
more ferrite --> softer, tougher, more ductile
cementite
- has an orthorhombic crystal structure
- chemical formula Fe₃C
- aka iron carbide
- forms from austenite, as when cooling the ferrite cannot absorb all the carbon and therefore it joins with iron to form cementite
- as carbon - more cementite
- more cementite --> harder, less tensile strength
pearlite
- lamellar structure
- when cooling from austenite the ferrite cannot absorb all the carbon so the carbon diffuses small distances to form cementite and ferrite layers
- steel is 100% pearlite at 0.83% carbon
Eutectoid Steel
0.83% carbon (100% pearlite)
Hypo-Eutectoid Steel
Less than 0.83% carbon (ferrite + pearlite)
Hyper-Eutectoid Steel
More than 0.83% carbon (pearlite + cementite)
(Forming Processes) Casting
Pouring molten metal into a cavity that produces an article shaped to specifications
(Forming Processes) Rolling
(Forming Processes) Extruding
(Forming Processes) Cutting
(Forming Processes) Joining
(Forming Processes) Fabricating
Isotropic
Strong in all directions
anisotropic
strong only in one direction