Unit Two Exam
1/398
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
399 Terms
What is transmembrane transport?
Makes use of membrane bound protein complexes to directly move specific proteins from one compartment to a topographically different compartment.
What is OMM?
The outer mitochondrial membrane (OMM) is the membrane that encases the mitochondrion, separating it from the cytoplasm and regulating the passage of molecules in and out of the mitochondria.
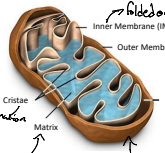
What is IMM?
The inner mitochondrial membrane (IMM) is the membrane that surrounds the mitochondrial matrix, playing a crucial role in the electron transport chain and ATP synthesis.
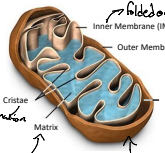
What is IMS?
The intermembrane space (IMS) is the space between the outer and inner mitochondrial membranes, playing a key role in the accumulation of protons during electron transport, which is essential for ATP production.
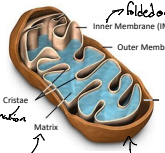
What is the matrix?
The matrix is the innermost compartment of the mitochondrion, containing enzymes for the Krebs cycle and mitochondrial DNA.
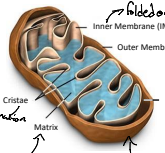
What is a cristae?
The cristae are the infoldings of the inner mitochondrial membrane that increase the surface area for ATP production. They house the components of the electron transport chain and ATP synthase.
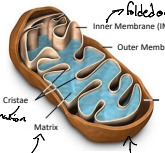
What is the order that a protein could enter the mitochondria; what are the layers?
OMM → IMS → IMM → Matrix
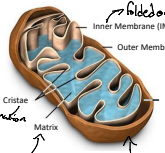
How many proteins does the mitochondrial DNA code for?
Mitochondrial DNA codes for 13 proteins, which are essential for mitochondrial function. These proteins are primarily involved in the electron transport chain.
How many proteins does the mitochondria use?
The mitochondria utilize over 1,000 different proteins, which are encoded by both mitochondrial DNA and nuclear DNA, crucial for its various functions.
If a protein needs to get to the matrix of the mitochondria what targets/sorting signals need to be present?
Only the Matrix Targeting Signal.

If a protein needs to get to the inner membrane of the mitochondria what targets/sorting signals need to be present?
The Matrix Targeting Signal and a Transmembrane Domain

If a protein needs to get to the inner membrane space or inner membrane of the mitochondria what targets/sorting signals need to be present?
Sorting Signal and The Matrix Targeting Signal

If the protein of interest is going to get through the inner membrane without a mitochondrial targeting signal. How does it work?
It has multiple transmembrane domains or sequences that encode for the mitochondria.

What are the translocators associated with a Matrix-destined protein that has only a matrix-targeting signal?
TOM complex and TIM23 complex
What are the translocators associated with an intermembrane-destined protein that has a matrix-targeting signal and a transmembrane domain?
TOM complex and TIM23 complex
What are the translocators associated with an intermembrane space and intermembrane-destined protein that has a matrix-targeting signal and sorting signal?
TOM complex and TIM23 complex
What are the translocators associated with an intermembrane-destined protein that has only transmembrane domains?
TOM complex and TIM22 complex
If a protein does not have a mitochondria localizing signal, how does it get to the mitochondria?
Chaperones are able to identify proteins without targeting signals and help in their transport to mitochondria.
For the proteins entering into the mitochondria there is typically a precursor protein. What is a precursor protein and what is the specific one for a mitochondrial protein.
A precursor protein is an inactive form of a protein that requires processing to become active. The specific precursor protein for mitochondrial proteins is the presequence which is a positively charged amphipathic alpha helix at the NH2 terminus. It directs the prevursor proteins to the inner membrane or matrix of the mitochondria.
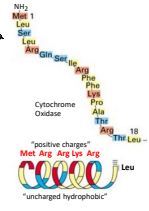
What happens to the 18-20 residue positively charged alpha helix in mitochondrial protein after the protein reaches the mitochondria?
After the protein reaches the mitochondria, the 18-20 residue positively charged alpha helix is cleaved off by processing enzymes, allowing the protein to fold into its active form.
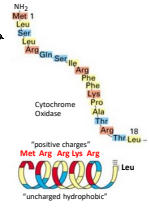
How far apart are the residues of positive charge in the mitochondria precursor protein to make the positive charge of the helix be one sided?
Every 4 to 5 residues.
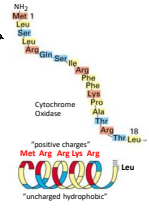
The precurssor protein sequence does not have to be an alpha helix. What is another example?
The beta barrel protein being targets to mitochondria by a dedicated beta-hairpin element. Known as the non classical signal
How do chaperones operate for a mitochondria protein?
The chaperones guide the polypeptide to the General Import Pore (GIP) and ATP hydrolysis facilitates the translocation of the protein into the mitochondria.
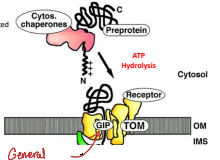
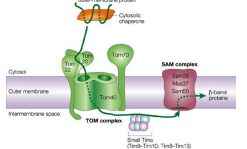
What does TOM stand for?
Translocase of the Outer Membrane
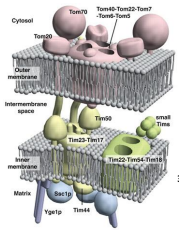
What does TIM stand for?
Translocase of the Inner Membrane
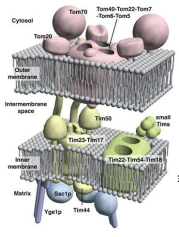
What are the 3 General Functions of TOM and TIM?
Receptor/Recognition
Channel Conductance
Force for Vectoral Movement
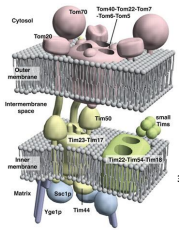
What is TOM40?
The Channel forming protein and main component of the TOM. complex of the OMM essential for import of protein precursor.
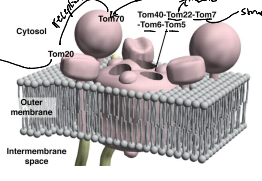
What is TOM22
Functions as the transit peptide receptor of the TOM complex, accepting precursor proteins from the TOM70 and TOM20
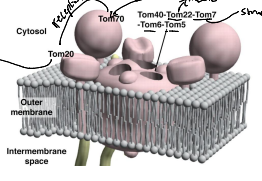
What is TOM5, TOM6, and TOM7?
They regulate the assembly an stability of the TOM complex.
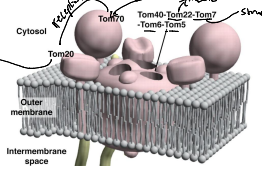
What is TOM70?
A receptor responsible for the recognition and translocation of chaperone assisted precursor proteins through he TOM complex of the OMM.
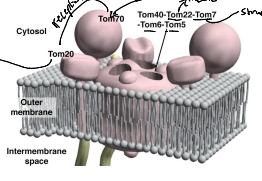
What is TOM20?
A receptor responsible for the recognition and translocation of pre-sequence containing proteins through the TOM complex of the OMM.
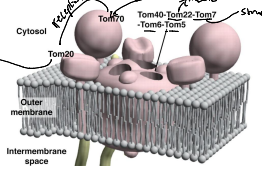
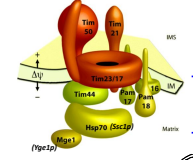
What is TIM23?
The essential component of ht eTIM23 complex, that mediates the translocation of pre-sequence containing proteins across the IMM.
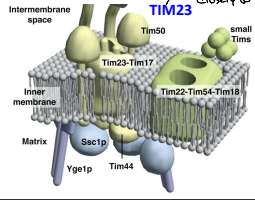
What is TIM17?
The essential component of the TIM23 complex, that mediates the translocation of pre-sequence containing proteins across the IMM.
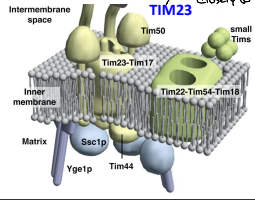
What is TIM44?
The component of the TIM23 complex sitting on the matrix side of the IMM which recruits Ssc1p and Yge1p to drive translocation into the matrix.
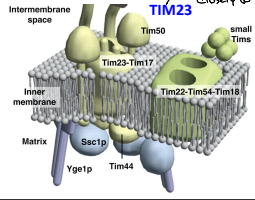
What is Ssc1p?
The mitochondrial HSP70 (mtHSP70) protein functioning in motor to drive translocation of pre-sequence containing proteins across the IMM.
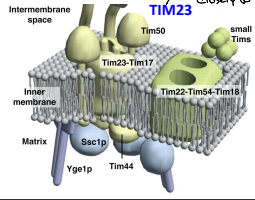
What is Yge1p?
The nucleotide exchange factor functioning in motor to drive translocation of pre-sequence containing proteins across the IMM.
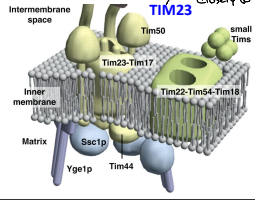
What is TIM50?
An essential for the translocation of pre-sequence containing proteins across the IMM to the mitochondrial matrix
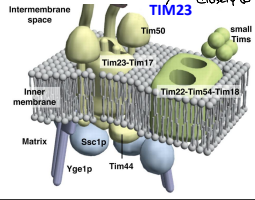
What is a homodimer?
A protein complex formed by two identical polypeptide chains that associate non-covalently. An example would be the TIM23-TIM23 Homodimer.
What is a Heterodimer?
A protein complex formed by two different polypeptide chains that associate non-covalently. An example is the TIM23-TIM17 complex.
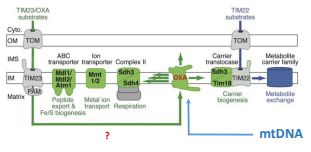
What is TIM22?
The essential component of the TIM22 compex, that mediates the translocation of proteins that DO NOT contain an N-terminal pre-sequence across the IMM.
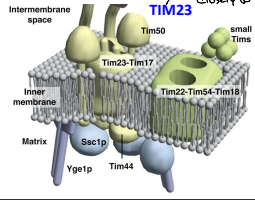
What is TIM54?
An essential component of the TIM22 complex that is believed to provide structural support to the complex.
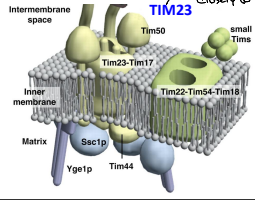
What is TIM18?
An essential component of the TIM22 complex that is believed to provide structural support.
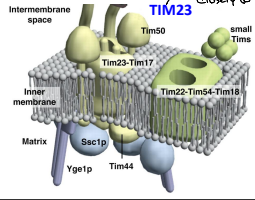
What are the Small TIM Proteins?
A group of proteins that function in helping to recognize non -pre-sequence proteins or internal localization sequence proteins for import through the TIM22 complex and some function in protein folding.
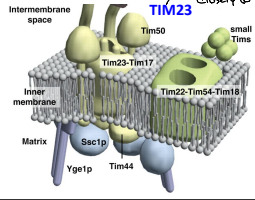
What is the contact site between IMM and OMM?
The site of contact between the inner and outer mitochondrial membranes that couples translocation through the TOM and TIM complexes.
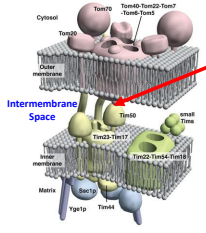
What does the TIM complex do during contact of the TOM and TIM complexes?
The TIM complex initiates a protein structure to act as a Pre-sequence binding sites and uses a protein to stabilize the inner and outer membrane by holding both membranes.
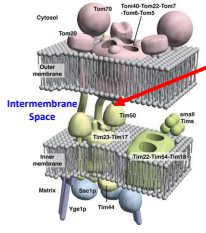
True or False: There is a membrane potential (∆ψm), which is a difference in electrical potential or voltage across a biological membrane, in the outer membrane of the mitochondria.
False; the outer mitochondrial membrane is not highly polarized.
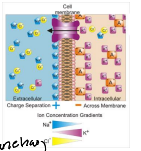
If an opening/pore exists in a membrane, then …
the membrane can not have membrane potential
What is mitochondrial processing peptidase (MPP)?
The enzyme that removes the signal peptide from mitochondrial preproteins after they are imported into the mitochondria.
There are two models for how mtHSP70 drives translocation: Thermal Ratchet Model and Cross Bridge Ratchet Model. What is the Thermal Ratchet Model?
The idea that each time a sufficiently long piece of the protein enters the matrix, a molecule of mtHSP70 binds to it in an ATP dependent manner in effect, using a hand over hand method of pulling the protein through the pore.
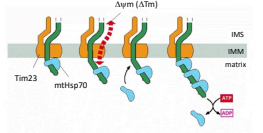
There are two models for how mtHSP70 drives translocation: Thermal Ratchet Model and Cross Bridge Ratchet Model. What is the Cross Bridge Ratchet Model?
The idea that as the protein enters into the matrix it is bound by mtHSP70. A conformational change to mtHSP70 works to actively pull the protein through the pore.
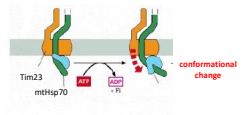
As an example of a portien without a primary localizing signal, there is AAC which is a mitochondrial carrier protein containing an Internal Localization Signal and interacts with the TOM70 receptor. What is protein(s) that move AAC to the TIM complex?
The Small TIMS (9, 10, 12) acitng as chaperones.
What is step one of AAC introduction to mitochondria?
The Biogenesis of AAC
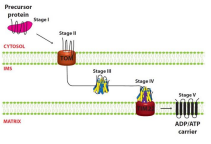
What is step two of AAC introduction to mitochondria?
The Precursor engages with TOM complex
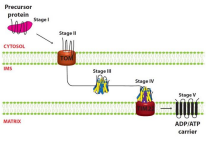
What is step three of AAC introduction to mitochondria?
The precursors enters IMS and binds with small TIMs — TIM9 and TIM 10 (chaperones)
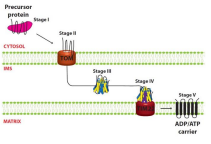
What is step four of AAC introduction to mitochondria?
The precursor interacts with TIM 12 and reaches TIM22 complex
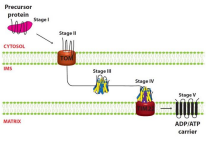
What is step five of AAC introduction to mitochondria?
The protein enters into TIM22 and becomes fully inserted into IM where it dimerizes into it fully functional form (ADP/ATP Carrier Protein)
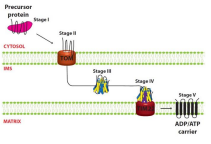
What is the Sorting and Assembly Machinery (SAM complex)?
The protein complex that operates after a preprotein has crossed the OMM, to mediate insetion of ß barrel proteins into the outer mitochondrial membrane. It ensures proper folding and assembly of these proteins into the membrane, facilitating their function.
What is the Presequence Translocase Associated Motor (PAM)?
The complex of proteins including mtHSP70 that drives translocation across the IMM and inserts proteins laterally into the IMM.
When trying to embed a protein in a membrane of the mitochondria, how is it done.
In both the outer and inner membrane the inner side of the membrane must be used to enter the membrane. `
What is the Oxidase Assembly Translocase (Oxa Complex)?
Required for exporting mitochondrially-encoded proteins and matrix imported proteins for insertion in the IMM. (This plays a back up role to PAM if necessary)
What is the Voltage Dependent Anion Selective Channel Protein (VDAC)?
A protein that regulates the flux of mostly anionic metabolites through the outer mitochondrial membrane and modulates interactions between mitochondria and other cellular components, playing a key role in metabolic regulation.
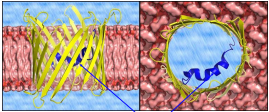
What is the reason that the OMM is neutral without a gradient?
VDAC
Where do mitochondria get most of their protein compliments from?
The cytosol.
How are proteins transported through the cytosol?
In a preprotein configuration by the action of either mitochondrial localization signals or by chaperones (or both).
What do pre-proteins pass through to reach the mitochondrial matrix?
A combination of TOM and TIM assemblies
What is a Terasaki ramp?
The connection layer between layers of the ER and the angle of this ramp is the same for all layers.
What is the starting point of newly synthesized proteins destined for the Golgi, endosomes, lysosomes, plasma membrane, or secretory vessels?
Endoplasmic Reticulum (ER)
Other than having synthesized proteins inside of the ER, what is actually synthesized mostly in the ER?
Most lipids, such as cholesterol and phospholipids
What ion does the ER sequester for use by the cell?
Calcium ions (Ca2+)
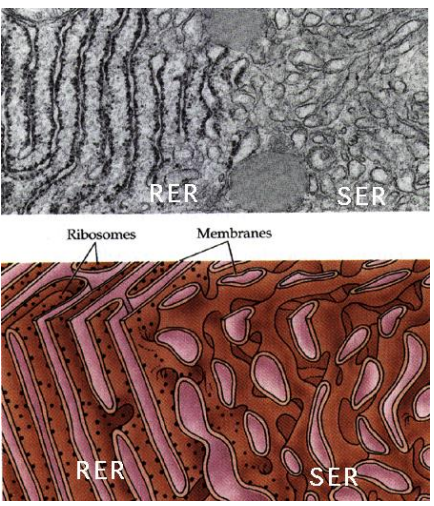
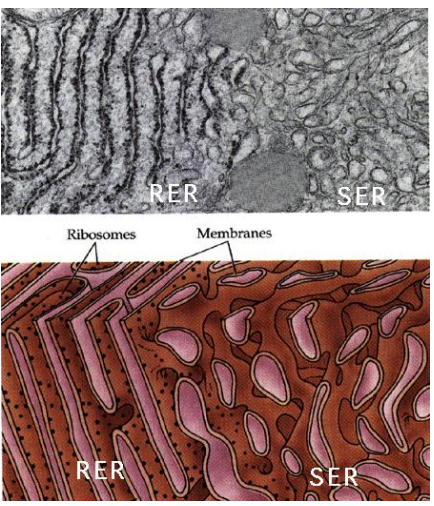
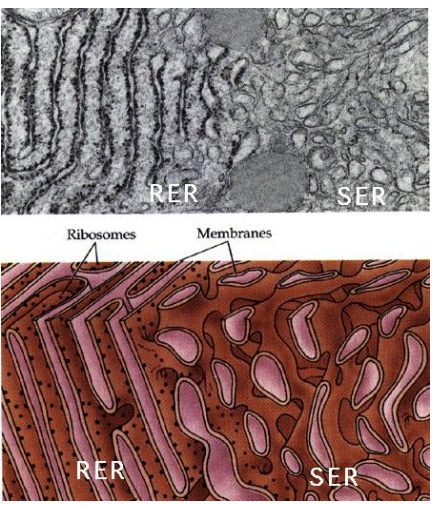
What part of the ER is coated in Ribosomes on the cytosolic side of the ER membrane, where protein synthesis and importing to the ER occurs?
Rough Endoplasmic Reticulum (RER)
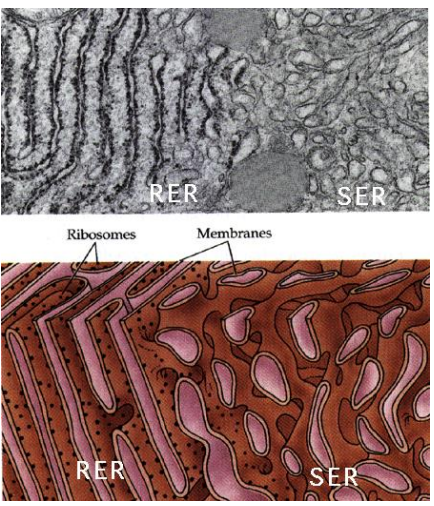
Why is ER structured versus the Smooth ER?
The structure comes from all of the proteins imbedded in the protein, which is not present in the Smooth ER which is free flowing due to lack of the proteins.
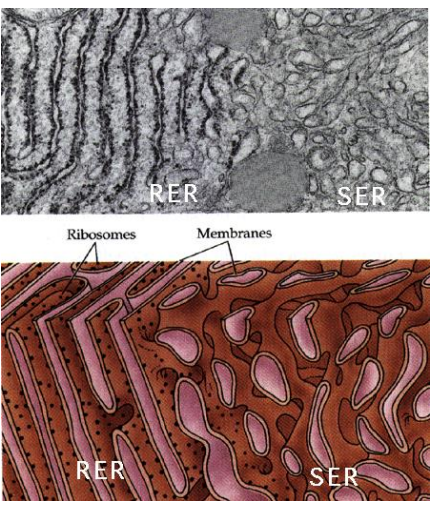
What part of the ER is lacking ribosomes and is the site of vesicle “budding” and lipid synthesis?
Smooth Endoplasmic Reticulum (SER)
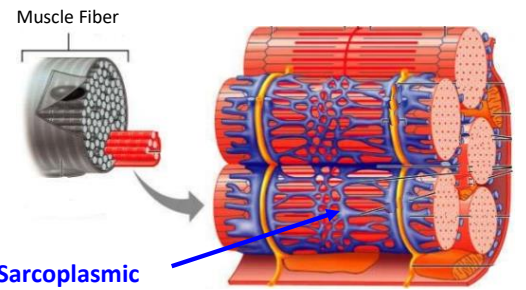
What is the specialized smooth ER bound to muscle fibers and also functions in storage of Ca2+ ions to be used when the muscle fiber is stimulated.
Sarcoplasmic Reticulum
True or False: The Endoplasmic Reticulum contains only Membrane Bound Ribosomes?
False; there are free floating ribosomes in the cytosol between layers of the ER.
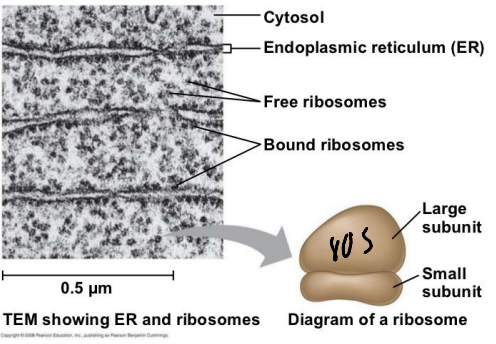
What is the ribosome in the ER?
The 80S Ribosome
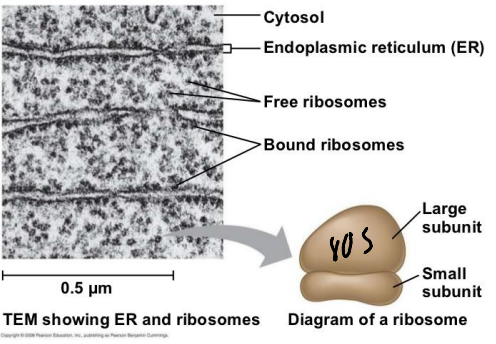
What is a ribosome that is attached to teh cytosolic side of the ER membrane and synthesize proteins destined for transport to the ER?
Membrane-bound Ribosome
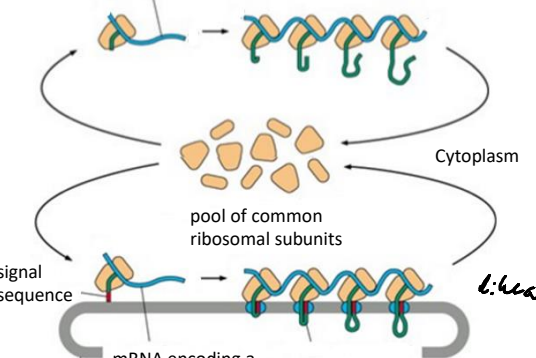
What is a ribosome that is unattached to any membrane and synthesize all other proteins from the nucleus?
Free Ribosome
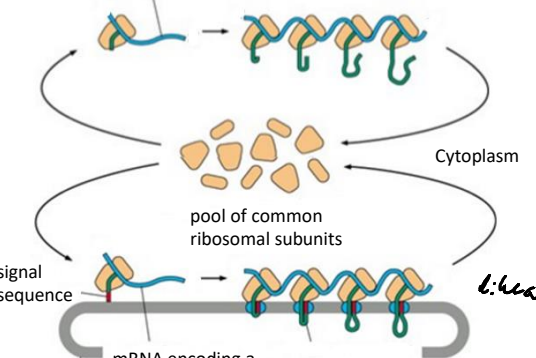
If an mRNA encodes for a cytosolic protein does it remain in the cytosol or go into the ER?
It remains in the cytosol, as free ribosomes synthesize cytosolic proteins.
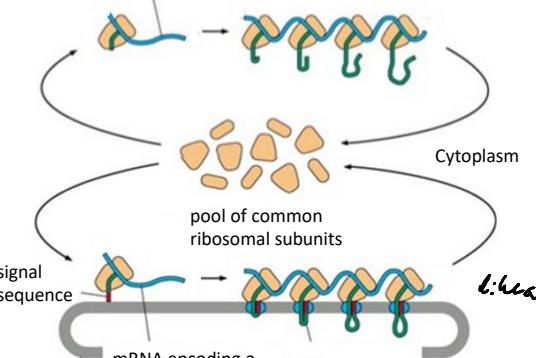
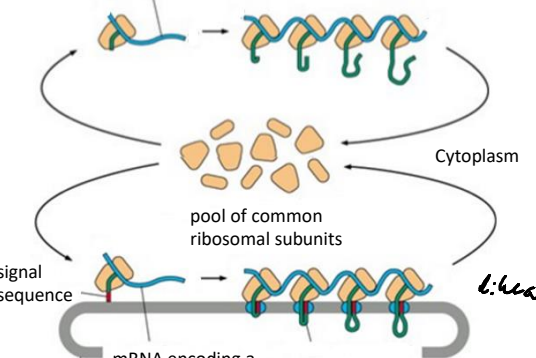
Using the pool of free ribosomal subuntis mRNA signal sequences are translated by poly ribosome complexes. What is a poly ribsome?
A complex of multiple ribosomes translating a single mRNA molecule simultaneously.
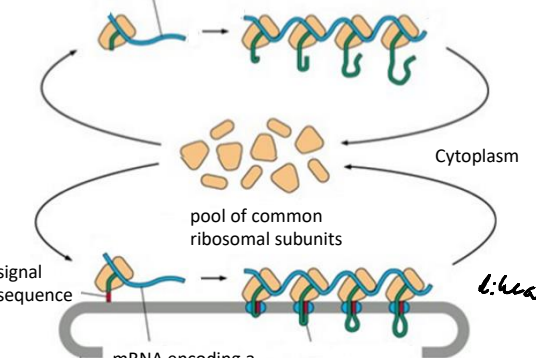
What do polyribosomes aid in?
They aid in the efficient synthesis of proteins by allowing several ribosomes to translate an mRNA strand at once, increasing protein production, as well as protecting the mRNA from degradation.
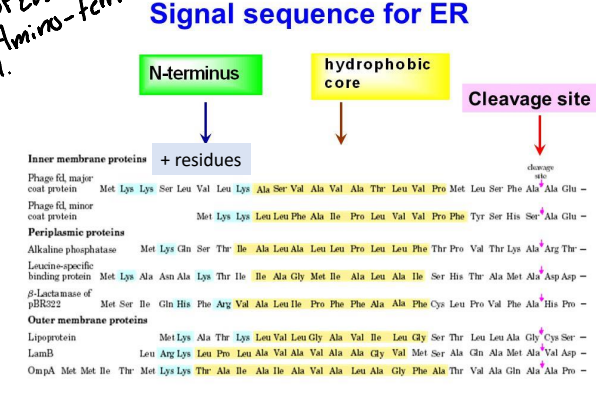
For the ER signal Peptide sequence it is typically found where?
Near the amino terminal but can be anywhere is the cell.
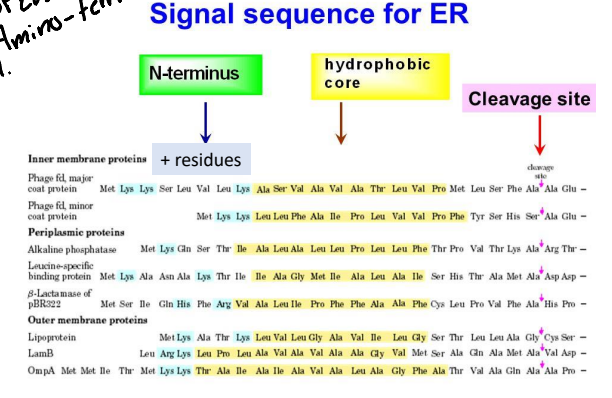
How long is the ER signal peptide sequence typically?
Typically 15-25 amino acids long.
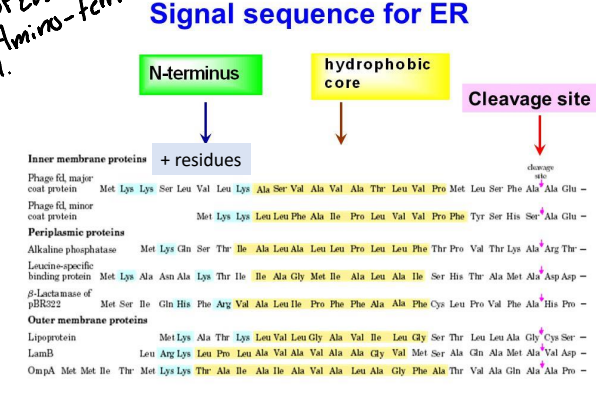
What is the chemical property of the ER signal peptide?
It is a very hydrophobic signal that is flanked on one side by charged residues.
What is the signal hypothesis in ER?
It describes how secretory and membrane proteins are targeted to the endoplasmic reticulum.
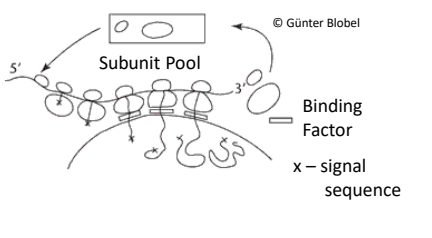
What is the first step in the signal hypothesis to get into the ER?
The ER signal emerges from the ribosome and directs the ribosome to a translocator protein on the ER membrane.
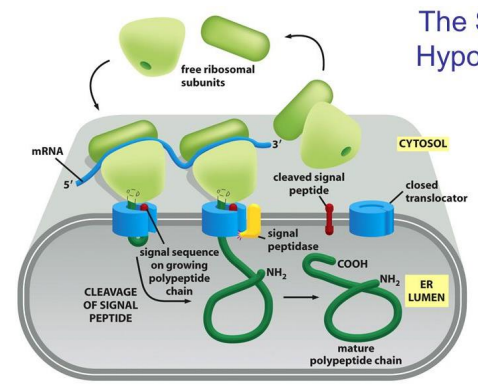
What is the second step in the signal hypothesis to get into the ER?
The polypeptide chain feeds into the ER lumen and the signal sequence is cleaved.
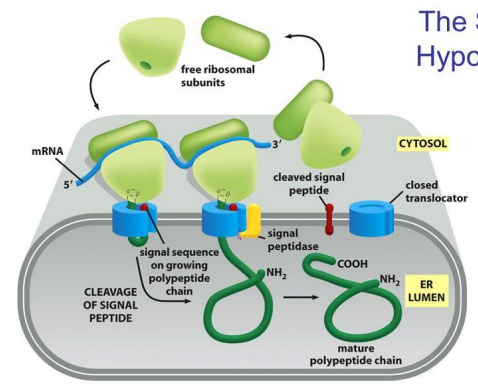
What is the third step in the signal hypothesis to get into the ER?
The mature polypeptide chain is released from the translocator after synthesis.
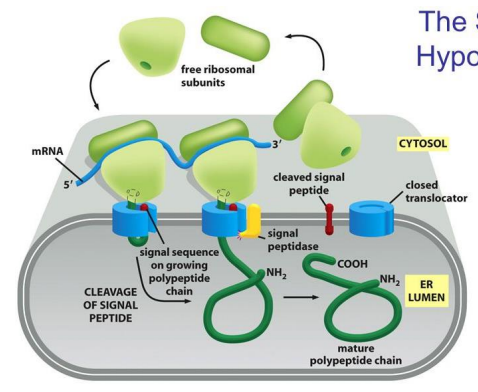
In the modern cell hypothesis of ER which is co-translational, what is the first step?
The 80S Initiation Complex forms in the cytosol
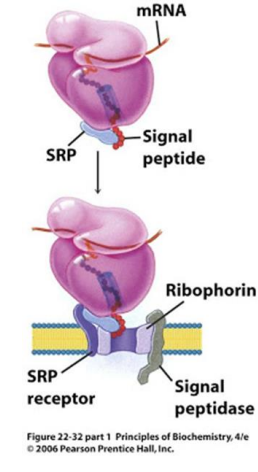
In the modern cell hypothesis of ER which is co-translational, what is the second step?
The signal peptide is synthesized by the ribosome at the N-terminus of the precursor.
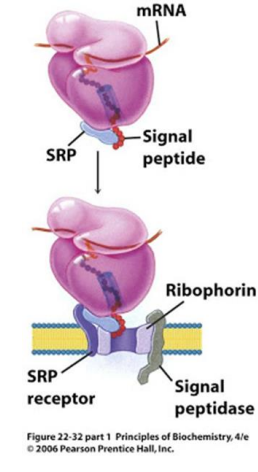
In the modern cell hypothesis of ER which is co-translational, what is the third step?
Once the signal peptide is made and extruded from the ribosome, it binds to the Signal Recognition Particle (SRP)
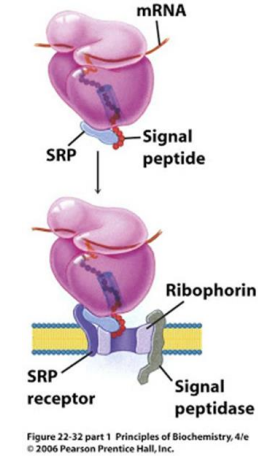
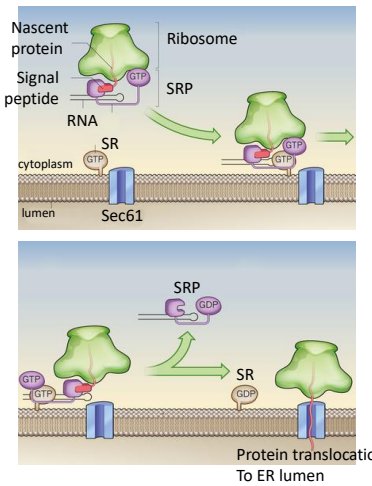
In the modern cell hypothesis of ER which is co-translational, what is the fourth step?
The SRP-Ribosome complex binds to the SRP Receptor Protein (SR) on the ER membrane.
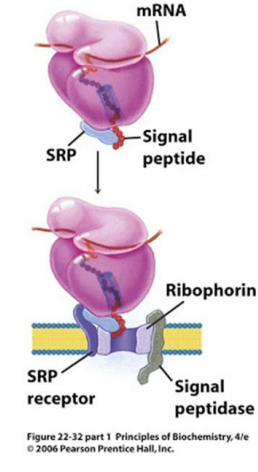
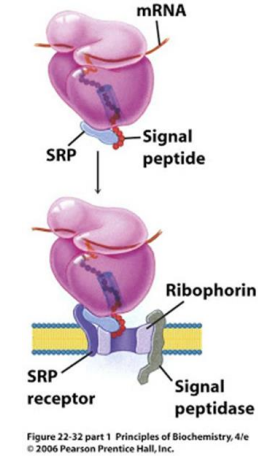
What is ribophorin?
A component of the ribosome that serves as a receptor for the signal recognition particle (SRP) during protein synthesis at the endoplasmic reticulum.
Are SRP and SR both ATPases, both GTPases, or One is an ATPase and the other a GTPase
Both are GTPases
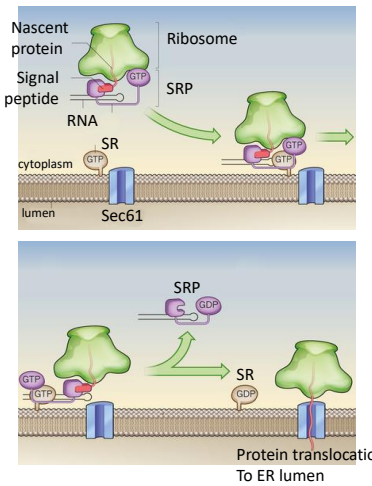
What is GTP hydrolysis required for, in order for the ribosome to interact in an SRP or SR?
The translocaton for the transfer of protein into the ER lumen.
In the modern cell hypothesis of ER which is co-translational, what is the fifth step?
The ribosome is anchored to the ER membranes by binding proteins called Ribophorins
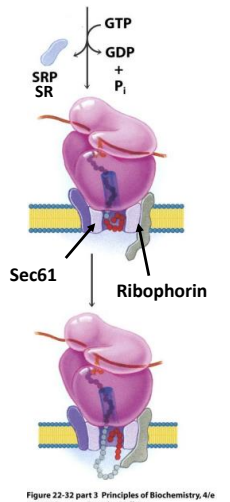
In the modern cell hypothesis of ER which is co-translational, what is the sixth step?
Signal peptide is inserted into the Translocase pore (Sec61 complex)
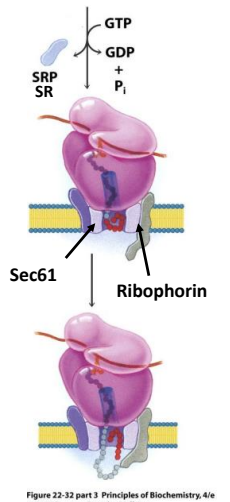
In the modern cell hypothesis of ER which is co-translational, what is the seventh step?
Once the ribosome complex is bound to the receptors proteins, SRP dissociates in a GTP hydrolysis reaction.
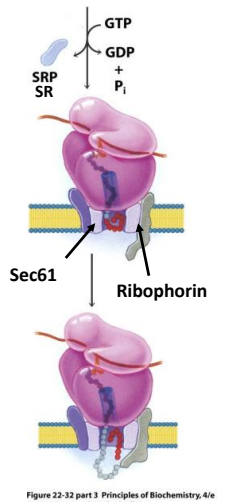
In the modern cell hypothesis of ER which is co-translational, what is the eighth step?
Translation resumes and the new polypeptide chain passes through the ER membrane.
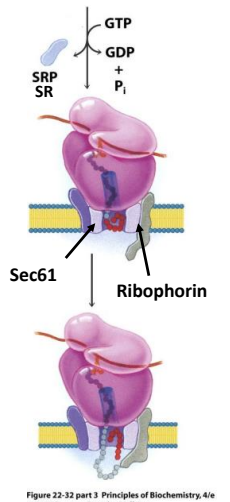
In the modern cell hypothesis of ER which is co-translational, what is the ninth step?
The signal peptide is cleaved by Signal Peptidase
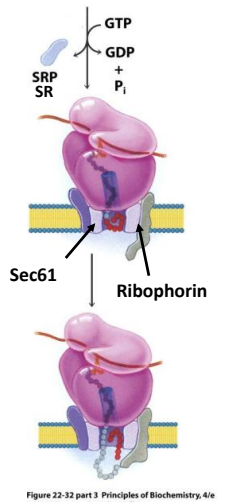
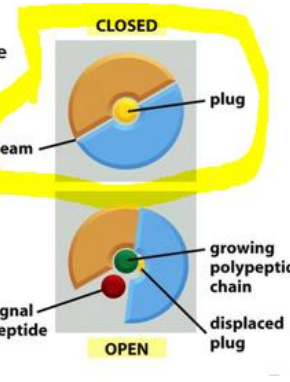
How does the Sec61 complex operate to allow the passage of one signal peptide at a time?
The complex first when closed operates as two functional units and a plug for the pore. Once opened the plug is moved to the side and the polypeptide can pass through. Once the signal peptide is through the complex changes shape opening on one side to allow the signal peptide to come up into the membrane. Once in the membrane the complex changes confirmation and is able to expel the signal peptide to the membrane to be destroyed.