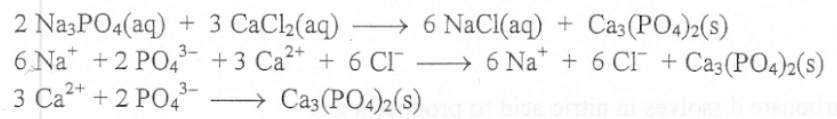Oxidation states and other important things
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
26 Terms
Seven Diatomic molecules (Example O2)
Nitrogen, Hydrogen, Oxygen, Fluorine, Chlorine, Bromine, Iodine
The positive ions are known as……..
Cations
The negative ions are known as ……………
Anions
Methane
Ethene
Ethane
CH4
C2H4
C2H6
Oxygen with its oxidation state
O2-
Silver with its oxidation state
Ag+
Zinc with its oxidation state
Zn2+
Aluminum with its oxidation state
Al3+
Hydrogen with its oxidation state when combined with non metal
Hydrogen with its oxidation state when combined with metal
H+
H-
Ammonia (Only chemical symbol)
NH3
Ammonium
NH4+
Hydroxide
OH-
Nitrate
NO3-
Sulfate
SO42-
Sulfide
S2-
Carbonate
CO32-
Hydrogen carbonate
HCO3-
Phosphate
PO43-
Chromate
CrO42-


If there is a compound named ……… acid then this element will always be in the compound.
Hydrogen (H)
Write the compound with its oxidation state
Practice
Hydrochloric Acid
Sulfuric acid
Nitric acid
HCl
H2SO4
HNO3
What is the state symbol for precitipate?
Solid
Sulfuric acid reacts to neutralise sodium hydroxide
What are the reactants?
H2SO4 and NaOH
Both of them.
What are these? And what happens when they react with water?
✅ Metal oxides (CuO, MgO)
✅ Metal hydroxides (NaOH, KOH)
✅ Ammonia (NH₃)
✅ Carbonates (CaCO₃, Na₂CO₃)
✅ Hydrogen carbonates (NaHCO₃, KHCO₃)
✅ Amines (CH₃NH₂, C₂H₅NH₂)
Bases

Practice
Answer