hubs191 immunology
1/82
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
83 Terms
what is immunology?
study of an organism’s defence system in health and disease
immune system composed of
organs (spleen)
cells (T-cells)
molecules (antibodies)
organised system that interact together to defend the body against disease
what are the main components of the immune system (lymphatic)?
tonsils
thymus
spleen
bone marrow
lymph nodes
includes primary and secondary lymphoid organs:
primary = where while blood cells made (bone marrow & thymus)
secondary = where these white blood cells turn into immune cells (spleen & lymph nodes)
what is special about the bone marrow in the lymphatic system?
primary lymphoid organ
source of stem cells that develop into cells of the ‘innate’ and ‘adaptive’ immune responses
what is special about the thymus in the lymphatic system?
primary lymphoid organ
‘school’ for white blood cells → T-cells
developing T-cells learn not to react to self
only 10% ‘graduate’ all others either cannot recognise pathogens or attack themselves, thus thymus has lots of dead/dying cells as well
what is special about the spleen in the lymphatic system?
secondary lymphoid organ
site of initiation for immune responses again blood-borne pathogens
no lymphatic drainage
what is special about the lymph nodes in the lymphatic system?
secondary lymphoid organ
located along lymphatic vessels → yes lymphatic drainage
lymph fluid from blood and tissue is filtered
site of initiation of immune responses
what are the 3 general ‘layers’ of protection in the immune system
chemical and physical barriers
innate ‘arm’
adaptive ‘arm’
what is involved in the first layer of the immune system: physical and chemical barriers?
skin:
made of top epidermis and inner dermis
epidermis contains lots of layers with the top layer being dead cells → cannot infect
dermis = thick layer of connective tissue and phagotic immune cells → hard to pierce through
phagotic cells will digest pathogens
also contains sweat glands = hypertonic
sebaceous gland = acidic (produce sebum)
lysozome = breaks down bacteria cell wall
mucosal layer:
1-2 layers
made of epithelium = tightly packed cells w/ mucus-producing goblet cells (alive)
line parts of the body exposed to air or lead to outside
traps and moves pathogens to be killed/removed
stomach - low ph
gull bladder - bile
mucus
defensins
lysozymes (only in tears/urine)
what is the mucociliary escolator?
inner
mucous gland (produce mucus)
basement membrane
columnar cell (have cilia attached)
goblet cell (secrete mucus)
cilia (beat & move mucus)
mucus (traps dust/pathogens)
(cilia move mucus up to the pharynx)
what is innate immunity/defences?
brute force immune cells
(includes skin/mucosal barrier)
already in place
rapid response - hours
fixed
limited specificites
has no specific memory
also includes internal defences:
phagocytes
natural killer cells
inflammation
fever
what is adaptive immunity/defence?
highly specialised response to pathogens
improves during the response
slow - days/weeks
variable
highly specific
has memory
includes:
humoral immunity: b-cells
cellular immunity: t-cells
what is the relationship between innate and adaptive reponses/immunity?
they are both intertwined and must work together - ‘two arms’
what is blood composed of?
plasma:
proteins
other solutes
water
cells:
platelets
white blood cells
red blood cells
what is important about bone marrow stem cells?
they are a source of blood cells as these hematopoietic stem cells can become any type of blood cell (hematopoiesis)
eg myeloid
red blood cells (erythrocytes)
granulocytes, monocytes, dendritic cells, platelets (innate immune cells)
lymphoid
T and B lymphocytes (adaptive immune cells)
what are granulocytes and how do they appear in blood vs tissue?
type of white blood cell that has granules filled with chemicals in the cytoplasm in which they can release
blood:
neutrophils: 75% of all leukocytes (WBC & type of granulocyte) and are highly phagocytic ‘eat and kill’ (numbers in blood increase during infection)
blood granulocytes can circulate in the blood and can move into tissue during inflammation
tissue:
mast cells: line mucosal surfaces (not in blood)
release granules that attract white blood cells to areas of tissue damage
what are the 2/3 types of phagocytic cells?
monocytes → macrophages:
in blood = monocyte = inactive (patrol)
once enters tissues = macrophage = active (high phagocytosis) ~ spleen/liver
macrophages can become resident or move through tissues
3 key functions:
phagocytosis
release of chemical messengers
shows pathogenic info to T cells (links innate and adaptive arms)
dendritic cells:
found in blood and all tissues exposed to the environment
found in low numbers but are very effective
phagocytic
most important cell type to help trigger adaptive immune responses
how do cells of the immune system move around the body?
cells are carried in the blood and in the lymph
cells can leave blood and enter tissues (specifically at site of infection)
lymph tissue collects into lymphatic vessels → these drain lymph into lymph nodes
how do innate cells recognise pathogens?
through pathogen-associated molecular patterns (PAMPs) and pattern recognition receptors
for viruses:
single/double stranded RNA
for bacteria:
cell wall
lipopolysaccharide
endotoxins
lipoteichoic acid
flagella
flagellin
nuclic acid
unmethylated CpG DNA
toll like receptors - pattern recognition receptors
function in phagocytic immune cells (macrophages/dendritic) to detect microbial components and trigger immune responses
can be on cell surface and recognise bacterial/yeast cell wall components
or inside cell in phagolysosome vesicle where they detect bacterial/viral nucleic acids
what happens during fever/pyrexia?
abnormally high temperature (37+)
resetting of body thermostat by hypothalamus
activated phagocytes/immune system produce pyrogens (‘fire generating’ cells) specifically pryogen interleukin-1 (after ingesting bacteria)
this is useful to the body because higher temp:
slows down microbial replication
enhances immune cell function
system reversed when
decreased phagocytosis (less microbes to be ingested)
decreased pryogen interleukin-1
decreased temperature
a common virus-associated pathogen associated molecule pattern (PAMP) is:
unmethylated CpG DNA
ds RNA
ss DNA
lipopolysaccaride/endotoxin
double stranded RNA
unmethylated CpG DNA = bacteria
ss DNA - not actually recognisable
lipopolysaccaride/endotoxin = bacteria
what are the 4 steps to the inflammatory response?
chemical signals from tissue-resident cells act to attract more cells to the site of injury or infection
Neutrophils enter blood from bone marrow (highly phagotic granulacytes) and cling to cell walls
chemical signals from resident cells dilate blood vessels and make capillaries ‘leakier’
neutrophils squeeze through the leaky capillary wall and follow the chemical trail to the injury site
what are the 5 stages of phagocytosis?
phagocyte adheres to pathogens or debris
phagocyte forms pseudopods (hug) that eventually engulfs the particles forming a phagosome
lysosome fuses with the phagocytic vesicle, forming a phagolysosome
toxic compounds and lysosomal enzymes destroy pathogens
sometimes exocytosis of the vesicle removed indigestible and residual material
(many myeloid cells are phagocytic)
how are phagocytosed microbes killed?
in the phagolysosome there is
a low pH environment
reactive oxygen (hydrogen peroxide) and reactive nitrogen intermediates (nitric oxide)
enzymes
proteases
lipases
nucleases
what is the complement cascade?
9 major proteins/complexes act in sequence to clear pathogens from blood and tissues
3 complement (C3) pathways
classical - complement binds to pathogen through antibody
alternative - complement binds directly to pathogen
lectin - complement binds to pathogen through a carbohydrate
1 of these pathways leads to all three effects (amplification) - C3 → C3a + C3b
label (opsonisation) - coating microbe with antibody or C3b fragment ~ C3b stays attached to pathogens
recruit - inflammatory mediators like C3a and C5(a) attract phagocytes to the site by inducing mast cells degranulation.
destroy - microbes coated with C3b are phagocytosed. the membrane attack complex (MAC) causes lysis through punching holes into the cell membrane → cell dies
what are the 3 main ways immune cells communicate with each other? (just names)
soluble molecules (cytokines or chemokines) binding to receptors on a cell membrane
cell surface-bound receptors binding to a cell surface-bound ligand
antigen (pathogen parts) being presented to cell surface bound receptors
how does ‘soluble molecules (cytokines or chemokines) binding to receptors on a cell membrane’ work for immune cell communication?
toll like receptors:
water soluble PAMPs (recognisable pattern part of pathogen) bind to TOL (pattern rec receptor) on surface which sends signal to nucleus
changes gene expression
cytokin receptor:
has extracellular/transmembrane/intercullular signalling (cytoplasmic tail) components
cytokine bonds to receptor, sending signal to nucleus which in/decreases gene expression
each cytokine has a specific receptor
chemokine receptor:
has extracellular/transmembrane/intercullular signalling (cytoplasmic tail) components
chemokine binds and sends signal to cell
always causes cell movement (toward higher chemokine concentration)
and sometimes changes gene expression as well/function of the cell
how does ‘surface bound receptors binding to surface bound ligands’ work for immune cell communication?
between T and B cells - alters function of one or both of the cells depending on if signalling parts are inside the cytoplasm or not
handshake - 1 has ligand, 1 has receptor
particular ligand to receptor
in/decreases regulation of gene transcription
how does ‘antigen being presented to a cell surface bound receptor’ work for immune cell communication?
between dendritic cell (or antigen presenting cell) and T-cell (B-cell can bind/recognise antigens directly)
dendritic cell shows antigen to T cell
activated t cell and function changes
gene transcription can change
increases regulation of their function
eg make more cyto/chemokines
make proteins to make then better killer cells
each T cell can only recognise 1 type of pathogen
antigen is presented through the MHC complex of the antigen presenting cell - there are 2 types
what is an antigen?
anything that has potential to be recognised by the immune system (B and T cells)
foreign antigen: anything from ‘outside’ (transplants, pathogens, some chemicals)
self-antigen: autoimmune issues
how do activated dendritic cells communicate with T cells?
activated dendritic (innate) cells:
make cytokines that bind to receptors on T cell membranes
cytokines key to send signals
have cell surface-bound receptors that bind to T cell surface-bound ligand (or vice versa)
present antigen to cell surface-bound receptors on T cells
this activates T cells = work better and/or grow/divide
example of innate and adaptive immune responses interacting
what are the 2 types of MHC complexes on antigen presenting cells?
MHC-1: presents endogenous (intracellular) antigens eg viruses
present on all nucleated cells
MHC-2: presents exogenous (extracellular) antigens
present only on antigen presenting cells
grab from outside and pulls it into the cell - dendrites best at this
what are cytokines and chemokines?
cytokines:
molecules such as interleukins and interferons that control growth and activity of immune cells
chemokines:
molecules that stimulate cell migration
both produced by innate and adaptive immune system cells and cells that influence the immune system eg epithelial cells
how do helper T cells activate B cells?
activated helper T-cells activated b cells by:
making cytokines that bind to receptors on B cell membranes
have cell surface-bound receptors that bind to a B cell surface bound ligand (or vice versa)
this communication leads to the activation of the B cell, and helps the B cell to make antibodies (essential for strong immune response against pathogens)
how does the complement cascade and B cells link innate and adaptive responses?
antibody produced by the B cell (adaptive) can bind to a pathogen → initiates complement cascade (innate)
and if complement fragments bind to a antigen, this can help activate B cells to produce more antibodies
(a cycle) - innate and adaptive immunity interacting
what is the big picture, 10 step process of the immune response when you step on a nail?
stand o nail, break physical barrier (skin)
pathogens enter the body
chemical mediators (produces by mast cells) leads to vasodilation and entry of phagocytic cells (neutrophils) to the tissue to ‘eat and destroy’
complement pathway triggered (but not classical as antibodies not yet produced)
dendritic cells in the skin become activated through recognition of pathogen associated molecular patterns (PAMPs)
dendritic cells move to local lymph node
once in lymph activated dendritic cells activate T cells via MHC
antigen + T cells and complement activate B cells
B cells produce antibody
complement, phagocytosis and antibodies help clear the pathogen
does volume of cells = function of cells in the blood?
no. white blood cells are a minor constituent of blood (buffy coat) but main cells involved in immunity
how does antigen sampling and presentation work (dendritic cells)
dendritic cells are present in major organs and can phagocytose the antigen
during this process they break it down into peptides (string on amino acids)
dendritic cells migrate from organs (eg skin) to draining lymph node
the present peptides on MHC protein complex to T cells (→ immune response begins)
how does adaptive immunity work using CD4 (helper T cells) and CD8 (killer T cells)?
step 1: pathogen/vaccine introduction
pathogens/vaccines introduce foreign antigens into the body
these are picked up by antigen presenting cells (dendritic cells)
cells process and display the antigen on MHC molecules (MHC2 for CD4+ and MHC1 for CD8+)
leads to helper or killer T cell activation (2 pathways)
step 2: T cell activation by APC
helper T cells recognise the antigens on MHC2 and become activated
or killer T cells recognise antigens on MHC1 - and with help of helper cells start process of becoming activated killer T cells
step 3a: helper T cells help B cells
B cells recognise native antigens
helper T cells interact and help B cells become plasma cells which produce antibodies (which neutralise pathogens) - final function
step 3b: helper T cells helps killer T cells
helps killer cells release cytokines (activates killer cells)
step 4: killer T cells kill virus infected cells
final function
what is the purpose of antigen uptake?
clearance of pathogens through the innate response
for presentation to T cells for initiating the adaptive response (and do something about the infection)
how did the adaptive immune response begin/evolve?
evolved 50 million years ago
phagocytes evolved to keep remnants of pathogens and display these to other cells of the immune system
all vertebrates and later discovered jawless fish have adaptive immunity (along with innate like everything else)
how does MHC1 molecules detect and respond to endogenous threats?
virus infects the cell
virus uses hosts machinery to make viral proteins in the cytoplasm
proteasome enzyme breaks down proteins into peptides (blender) & tagged for immune display
peptide transport to ER
peptides moved into the ER where MHC1 molecules are being assembled
MHC1 loading
inside ER, peptide is loaded onto a MHC1 molecule and this complex is shipped to the cell surface
killer T cells then recognise and initiate response
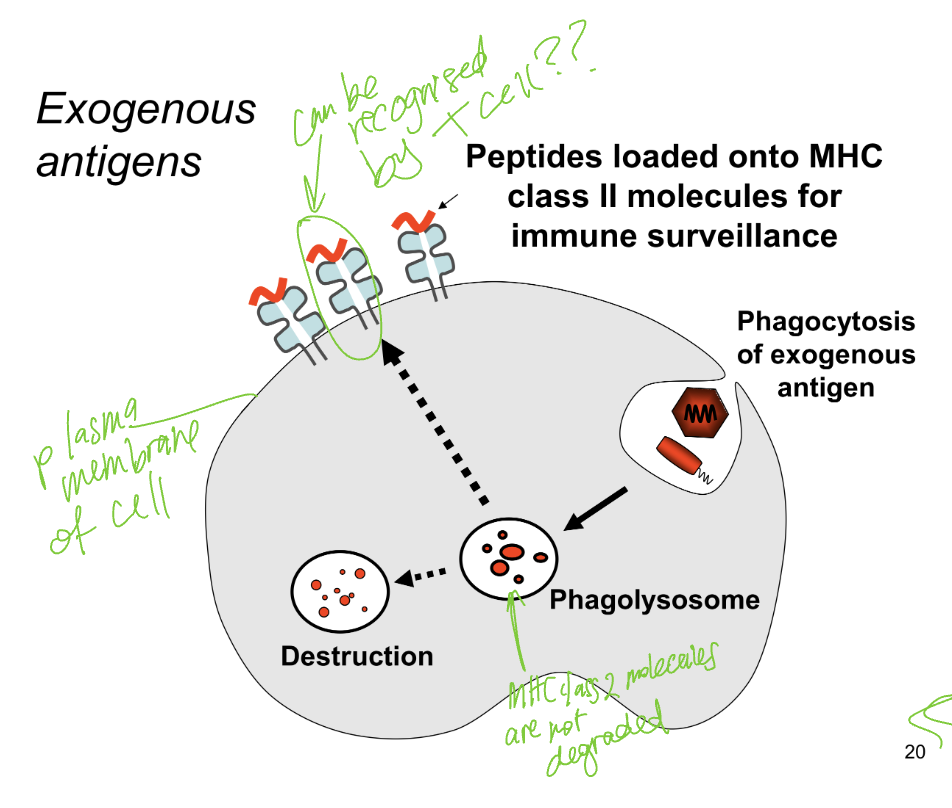
how does MHC2 molecules detect and respond to exogenous threats?
phagocytosis
antigen presenting cell engulfs an exogenous antigen and forms vesicle inside cell
phagolysosome formation
formed and antigen broken down into peptides
peptide loading on MHC2
MHC2 not degraded in phagolysosome but are waiting to bind onto broken down peptides → complex is formed
transport to cell surface
complex is transported to plasma membrane of the antigen presenting cell and is displayed on the surface
recognition by helper T cells
helper T cells scan the MHC2 molecules - if the peptide is foreign the helper gets activated and begins immune response
what are T cells?
lymphocytes that arise in the bone marrow and fully develop in the thymus
T cells express T cell receptor (TCR) with co-receptors (either CD4 - helper or CD8 - killer)
when then are fully mature they can recognise MHC/peptide complexes
how do T cells develop their T cell receptors? TCR
through thymic gene rearrangement:
immature T cells start in the bone marrow - they are germline (untouched) and cannot recognise antigens
TCR gene rearrangement happens in the thymus - the rearrangement is random and causes each T cell to only express one type of receptor as mature/naieve T cells
this creates diversity in T cell repertoire
how do T cells recognise antigens?
they recognise specific antigens (peptides) presented to them on MHC (cell surface proteins) on antigen presenting cells
the variable region of the T cell binds to the peptide/MHC complex
T cell receptors need both peptide and MHC to recognise and bind
how do co-receptors help T cells
2 types of co-receptors that help T cells bind properly
CD4 co-receptor helps T cell receptor bind to MHC class 2
CD8 co-receptors hep T cell receptors bind to MHC class 1
immature T cells express both CD4/8 when developing, when in thymus they may interact with thymic epithelial cells that present MHC molecules. if it binds with MHC class 2 then CD4 stays and becomes CD4+ (helper) T cell - and vice versa
what is the difference between CD4 and CD8 expression on mature T cells?
CD4+ (helper) T cells recognise antigens presented by MHC-2 (typically on antigen presenting cells like dendritic cells)
CD8+ (killer) T cells recognise antigens presented by MHC-1 (typically found on all nucleated cells
(therefore dendritic cells have both MHC1and 2)
what are activated and non-activated T cells called?
immature - germline cells (before receptor differentiation)
differentiated, non-activated by MHC/peptide = naive
fully activated = effector T cells
what does a CD4 helper T cell do?
recognises MHC-2 /peptide
helps CD8 (killer) T cells become cytotoxic
helps B cells make antibody
what does a CD8 killer T cell do?
recognises MHC-1/peptide
develops into cytotoxic T lymphocyte’ (CTL) or cytotoxic T cell
triggers apoptosis (programmed cell death) in the infected cell
how are cytotoxic T cells activated?
An antigen-presenting cell (APC) (like a dendritic cell) displays a viral or pathogenic peptide on MHC Class I.
A CD8+ T cell recognizes this via its TCR + CD8 co-receptor.
also:
A CD4+ helper T cell (activated via MHC Class II on the same APC) secretes cytokines.
These cytokines act as activation signals to help the CD8+ T cell fully activate.
how does a cytotoxic T cell kill infected cells?
it can recognise virally-infected cells that display viral peptides on MHC Class I.
it binds to this using its T cell receptor and CD8 co-receptor
this triggers apoptosis (programmed cell death) in the infected cell
what are memory T cells?
forming effector cell also causes formation of memory T cells (T cells form memory cells)
memory CD4 or CD8 T cells reside in the body for long periods of time
memory T cells become effector cells much quicker than naive T cells
where are B cells made and trained?
both in the bone marrow
what is a B cell receptor made up of ?
2 identical light and heavy chains in a Y shape. these make up 2 identical antigen binding sites
chains connected by disulfide bridge
contains variable and constant regions
spans past transmembrane region and into cytoplasm of B cell
surface of B cell covered with ~1000,000 BCR - mostly IgM and IgD antibodies
what is the function of the B cell receptor
BCR binds antigen and (partially) activates the B cell
surface of B cell covered with ~1000,000 BCR - mostly IgM and IgD antibodies
BCR is anchored on the membrane via transmembrane domain
secreted antibodies from the same B cell differ as they lack a transmembrane domain
what are the 3 functions of antibody?
neutralisation
antibodies cover/block receptors on the virus/pathogen so they cant hurt us
(can also occur to bacterial toxins)
opsonisation
antibodies coat the microbe so that they make it easier for phagocytes to eat
can also help by catching motile microbes
complement activation via classical pathway
leads to more opsonisaition
recruitment - more phagocytosis
MAC - causes microbe to leak fluids and die
how do antibodies bind to antigens?
through the antibody binding site - epitopes
antibodies bind to native (unprocessed) antigens
several different antibodies may target a single type of microbe
what are the 5 main different types of antibodies? (in order of abundance)
IgG - monomer
IgA - dimer
IgM - pentamer
IgE - monomer
IgD - monomer
what are the features of the IgG antibody?
Distribution:
most abundant Ig class in the blood
Function:
opsonises/neutralises
only Ig class that crosses the placenta therefore provides passive immunity
targets virus/bacteria
what are the features of the IgA antibody?
Distribution:
present in secretions such as tears, saliva, mucus, and breast milk
monomeric form in blood
Function:
defence of mucous membranes eg gut (protects against invasion)
present in breast milk therefore provides passive immunity on nursing infant
targets virus/bacteria
what is passive immunity?
in early years of a baby’s life they receive immunity through the mother while theirs are still developing
through the placenta - IgG antibody
through breast milk - IgA antibody
what are the features of the IgM antibody?
Distribution:
First Ig class produced after initial exposure to antigen
expressed on naive B cells (low concentration in the blood)
Function:
very effective in activating complement (classical)
targets extracellular bacteria
acts as B cell / antigen receptor (together with IgD)
what are the features of the IgE antibody?
Distribution:
present in blood in low concentrations
Function:
evolved for immunity to multicellular parasites
often causes allergic reaction responses
activates mast cells for parasite immunity and the allergic response
what are the features of the IgD antibody?
Distribution:
expressed on naive B cell
not high concentration in blood
Function:
together with IgM, acts as B cell / antigen receptor
specific function unknown
how is a naive B cell activated and what does it form?
activated by both cytokines from helper T cells and binding to naive antigen
forms:
plasma cells - antibody factory
small number of memory B cells
what are memory B cells?
formed when B cell is activated
memory cells persist for years in blood and lymphatic tissue
expresses antibody as B cell receptor but does not secrete antibody
responds rapidly to antigen encounter and becomes plasma cells (→ then secretes antibodies)
what is the primary immune response?
first time body (specifically adaptive immune system) encounters particular pathogen:
takes 7-14 days for sufficient antibody is produced to eliminate pathogen (slow response)
relatively low amount of antibody produced - mainly IgM
what is the secondary immune response?
second+ time body (specifically adaptive immune system) encounters particular pathogen:
relies on memory B cells
takes 2-3 days for sufficient antibody to eliminate pathogen is produced - fast
high amount of antibody produced, now mainly IgG with some class switching to IgA and IgE (but in low levels)
different antigens protect different surfaces
more specialised than primary response
what is severe combined immunodeficiency disorder? SCID
recessive X chromosome link disease (thus, genetic)
therefore more common in males- females are carriers
patients lack functional T and B cells → no adaptive immune response
how can your immune response be suppressed through viruses?
measles, HIV, and many other viruses interfere with normal host immune system
HIV targets and can kill CD4 T cells
without treatment this leads to diminished levels of CD4 T cells unable to provide help for antibody and cytotoxic responses (no activation of B and T killer cells)
how does HIV specifically lead to a compromised immune system
HIV has has a receptor that targets the CD4 T cell
infection leads to loss of CD4 T cells
CD4 T cells help both antibody and cytotoxic responses
thus, HIV infection impacts on immunity to microbes (fungi/bacteria/viruses) and to cancer
how is autoimmune disease normally prevented?
host mechanism of immune tolerance:
screening of T and B cells before they are fully developed (tests at the school)
this occurs in the thymus (t) and bone marrow (b)
thymus acts to delete auto-reactive T cells
there are also periphery mechanisms that act to get rid of faulty cells outside of the primary centers
failures in immune tolerance → autoimmunity
other triggers could be from:
innate system (triggers or exacerbate)
genes
autoimmune attack is mediated by the adaptive immune response
what are 2 examples of autoimmune conditions?
rheumatoid arthritis: affects joints
autoreactive T and B cells attack self-antigens present in the joints
causes inflammation → joint remodelling → deformation of joints
affects 1% of the population and has onset later in life
type 1 diabetes:
insulin beta cells attacked (very specific)
prevents production of insulin
how do allergic reactions occur? (peanut)
dendritic cells preset peptides from peanut proteins (allergens) to CD4 helper T cells
primed helper T cells activate B cells to secrete IgE antibody (after class switching)
secreted IgE binds to mast cell receptors (Fc Receptor) - only mast cells have FC receptors specific to IgE
binding of peanut proteins to FcR on mast cells (plastered with IgE) triggered mast cell degranulation and release of histamine and other inflammatory mediators
systematic symptoms: airway obstruction/hives/low BP
local symptoms: physiological responses to get rid of parasite (nausea/diarrhoea/swelling)
what is the one random peanut fact he told us was important?
more than 2% of people in the US have a peanut allergy
this rate has quadrupled in from 1997-2010
how does antigen bind to the Fc receptors in a allergic response?
Fc receptors on mast cells bind to the Fc domain (on the constant region) of the IgE antibody
the FcR facilitates a number of functions, including phagocytosis and mast cell activation
what is the order of events for changes in antibody structure (class swtiching)
early: (during generation of diversity in bone marrow)
occurs in developing B cell before they encounter an antigen
the variable regions (top of Y) are randomly assembled through gene rearrangement creating unique binding sites
late: (class switching during immune response)
after B cell is activated by an antigen and moves to a lymph node it can change its constant region (bottom of Y)
this is called class/isotype switching (eg initially: IgM→IgD→IgG→IgA→IgE for allergy response)
what broad two players are responsible for adaptive immunity?
cell mediated immunity and antibody production
how does a cytotoxic T cell cause apoptosis?
using perforin - which forms pores in the target cell, and granzyme - which can now enter the cell and triggers cell death
what is clonal selection/expansion?
for B and T cells:
selective expansion (cell division) of white blood cell at interact with that specific antigen/peptide
the other cells that are not involve just ‘sit there chilling’
this allows the body to only produce antibody for the specific pathogen it recognises (immune response is very powerful and can do damage)
what different forms of vaccines were we told about?
Live attenuated
eg mumps, measles, rubella, polio-sabin
live but moderated
Killed
eg polio-salk, some covid and flu vaccines
Sub-unit protein
eg tetanus, covid
contains only parts of the pathogen so is harmless to cells but still has everything to trigger immune response
may need help to trigger immune response with adjuvants
Sub-unit mRNA
eg covid
in the body are translated into proteins that trigger the immune response
may need help to trigger immune response with adjuvants
what are adjuvants for vaccines?
immune stimulants added to vaccines that enhance the activation of antigen presenting cells (because vaccine is missing PAMPs to trigger the immune response)
eg mRNA SARS-2 vaccine is intrinsically adjuvinated: the lipid-encapulated mRNA is immunostimulatory
RNA can stimulate Toll-like recpetors