Bio Final-all units
1/357
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
358 Terms
Covalent bonds
type of strong chemical bond where two atoms share one/more pairs of valence electrons
electronegativity
measure of atoms attraction for shared electrons
nonpolar covalent bond
electrons are shared equally between 2 atoms of similar electronegativity
polar covalent bonds
atoms differ in electronegativity. Shared electrons are pulled closer to the more electronegative atom making it slightly negative and the other atom slightly positive
hydrogen bonds
weak chemical bond formed when a slightly positive hydrogen of a polar covalent bond in one molecule is attracted to the slightly negative atom of a polar covalent bond in another molecule
polar molecules
molecule containing polar covalent bond and having an uneq1ual distribution of charges in different regions of the molecule
denaturation
where a protein unravels, can be because of pH, salt, or heat
gene expression
genetic information flows from genes to proteins, the flow of genetic information from the genotype to the phenotypes
competitive inhibitor
if it fits, it sits
Reversible competitive inhibitor
can interact and detach as much as they want
irreversible enzyme competitive inhibitor
gets attached by covalent bond and never detatches
competitive reversible inhibitor
attaches at active site and blocks the substrate
noncompetitive reversible inhibitor
changes the shape of the active site reducing the ability to bind
Oxidized
lost electrons, NAD+, ready to accept electrons
reduced
has electrons, NADPH, has electrons
Stage 1 Light Reactions (thylakoid membrane)
Light + H2O → O2 + ATP +ADPH
water is split → releases electrons and O2
Light energy excites electrons → transferred from NADP+ to NADPH
ATP is generated from ADP+Pi
Stage 2 Clavin Cycle (stroma) (light independent reactions)
CO2+ATP+NADPH → Sugar(G3P)
Carbon fixation: CO2 incorporated → organic compounds
molecules reduced to form sugars
powered by ATP+NADPH from light reactions
doesn’t require light but depends on the light reactions
The two alleles an individual rabbit has for the FUZY gene
a. are always the same.
b. are always different.
c. can be either the same or different.
c. can be either the same or different
Within a population of rabbits, can there be more than two different alleles for the FUZY gene?
No
Yes
Yes
Expression of the human gene NCR produces a protein that’s important for controlling cell division. A mutation in the NCR gene that increases the activity of the NCR protein has been linked to various types of cancer. Therefore, it’s reasonable to predict that the NCR protein __________ cell division.
promotes
inhibits
promotes
A diploid cell has twenty total chromosomes (pieces of DNA). Therefore, the cell has _____ pairs of homologous chromosomes prior to DNA replication, and _____ pairs of homologous chromosomes after DNA replication.
ten; twenty
twenty; twenty
ten; ten
twenty; forty
ten; ten
Homologous chromosomes _____________ genes; sister chromatids _____________ genes.
a. can have the same or different; always have the same
b. can have the same or different; can have the same or different
c. always have the same; can have the same or different
d. always have the same; always have the same
d. always have the same; always have the same
Homologous chromosomes _____________ alleles for a gene; sister chromatids _____________ alleles for a gene.
a. can have different; can have different
b. always have the same; can have different
c. can have different; always have the same
d. always have the same; always have the same
c. can have different; always have the same
In a human lung cell, a substitution mutation occurs in a gene on one chromosome; the other homologous chromosome is not affected by the mutation. This results in a lung cell with one mutated copy of the gene and one non-mutated copy of the gene. What will be found in the daughter cells when this cell divides by mitosis?
Hint - Think about the chromosomes present in a diploid cell and what you know about the process and products of mitosis.
a. both daughter cells will have two mutated copies of the gene
b. both daughter cells will have one mutated and one non-mutated copy of the gene
c. one daughter cell will have two mutated copies of the gene, and the other daughter cell will have two non-mutated copies of the gene
d. one daughter cell will have one mutated copy and one non-mutated copy of the gene, and the other daughter cell will have two non-mutated copies of the gene
b. both daughter cells will have one mutated and one non-mutated copy of the gene
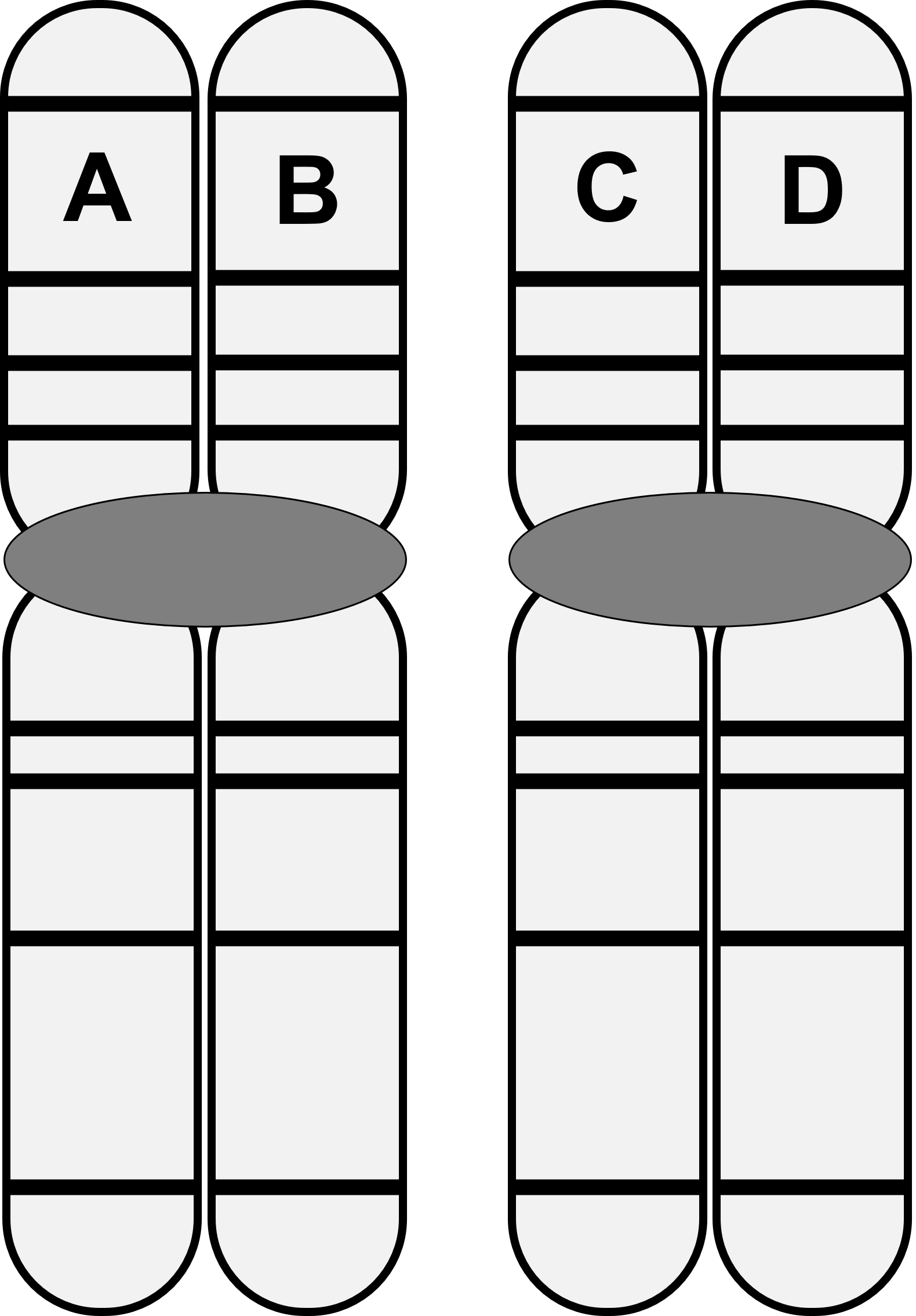
The image below shows a pair of homologous chromosomes following DNA replication. Letters indicate individual pieces of DNA.
What letter pairs represent non-sister chromatids?
A and C
A and D
B and C
B and D
All of the above
All of the above
Non-sister chromatids
a. always have the same genes and the same alleles for each gene.
b. can have different genes.
c. always have the same genes, but can have the same or different alleles for each gene.
c. always have the same genes, but can have the same or different alleles for each gene.
Which of the following, if any, correctly describes a difference between prokaryotic and eukaryotic cells/organisms?
a. All prokaryotic cells are haploid; all eukaryotic cells are diploid.
b. Prokaryotic cells only divide by mitosis; eukaryotic cells only divide by meiosis.
c. Prokaryotes only reproduce asexually; eukaryotes only reproduce via sexual reproduction.
d. None of the above
d. None of the above
A diploid cell has twelve pairs of homologous chromosomes. How many individual chromosomes (pieces of DNA) will be copied during DNA replication?
six
twelve
twenty-four
forty-eight
twenty-four
Select ALL statements that correctly describe BOTH prokaryotic division and mitosis.
a. Nuclear envelope (membrane) breaks down.
b. Each copy of a chromosome generated by DNA replication is distributed to opposite ends of a dividing cell.
c. Produces two genetically identical daughter cells.
d. DNA is replicated once prior to division.
b. Each copy of a chromosome generated by DNA replication is distributed to opposite ends of a dividing cell.
c. Produces two genetically identical daughter cells.
d. DNA is replicated once prior to division.
Compare the purposes of cell division in prokaryotes and eukaryotes.
In Prokaryotes there is one circular chromosome and are not wound around proteins, there are no organelles, and there are ONLY single-celled organisms.
In Eukaryotes there are multiple linear chromosomes that are wrapped around proteins, the chromosomes are in the nucleus, and there are both single-celled and multiple celled organisms.
Describe the differences between prokaryotic and eukaryotic cells/genomes that contribute to differences in the purposes and processes of cell division.
Prokaryotic cells have a singular circular DNA molecule and are divided by asexual reproduction while eukaryotic cells have multiple linear chromosomes within a nucleus and divide by mitosis or meiosis for growth, repair, or sexual reproduction.
Describe the process of prokaryotic cell division.
Prokaryotic cell division occurs through asexual reproduction, whether the circular DNA replicates, the cell elongates, and the plasma membrane pinches inward to form two genetically identical daughter cells.
Are homologous chromosomes present in a diploid cell prior to DNA replication? What about sister chromatids?
Yes, homologous chromosomes are present in a diploid cell prior to DNA replication, but sister chromatids are not formed until after DNA replication occurs.
A diploid cell has 14 different chromosomes. How many individual chromosomes (pieces of DNA) are in the nucleus? What about a haploid cell with 14 different chromosomes?
A diploid cell with 14 different chromosomes has 28 individual chromosomes in the nucleus, while a haploid cell with 14 different chromosomes has 14 individual chromosomes.
Do two homologous chromosomes always have the same genes? The same alleles for each gene?
Two homologous chromosomes always have the same genes in the same order, but they have different alleles for those genes.
Elephants are diploid organisms. Can more than two alleles for a particular gene exist in an elephant population? Can an individual elephant have more than two alleles for a particular gene? Can an individual elephant have only one allele for a particular gene? Explain your answers.
Yes, more than two alleles for a particular gene can exist in an elephant population, but an individual elephant can have only alleles for that gene-one from each parent-unless the gene is on a sex chromosome, in which case an individual may have only one allele if they have a single copy of that chromosome.
Explain why it’s necessary for eukaryotic chromosomes to condense prior to mitosis. Explain why condensing of chromosomes prior to division is not necessary in prokaryotic cells.
Eukaryotic chromosomes must condense before mitosis so the long strands of DNA can be organized and separated accurately without tangling or breaking. In prokaryotic cells, the DNA is much smaller, circular, and not contained in a nuclus, so it can be separated easily without the need for condensation.
Describe the steps of mitosis. Draw and label pictures to illustrate your description Keep it simple and stick to a diploid cell with two different chromosomes (so four pieces of DAN total). You could also try drawing it out for a haploid cell with three different chromosomes.
1. Interphase-the cell prepares for division by copying its DNA and organelles; chromosomes are not yet visible
2. Prophase-chromosomes condense and become visible, and the nuclear membrane starts to break down.
3. Metaphase-chromosomes line up in the middle of the cell, attached to spindle fibers
4. Anaphase-sister chromatids are pulled apart to opposite sides of the cell
5. Telophase-two new nuclei firm around the separated chromosomes as the cell begins to split
6. Cytokinesis-the cytoplasm divides, forming two identical daughter cells.
Humans have twenty-three pairs of chromosomes. During division of a human skin cell, the two sister chromatids of one replicated chromosome fail to separate. How many individual chromosomes will each of the daughter cells have?
If the sister chromatids of one replicated chromosome fail to separate during division, one daughter cell will get 47 chromosomes(one extra) and the other will get 45 chromosomes(one missing), instead of the normal 46.
A mutation in the gene PTEN has been linked to cancer. The mutation increases the activity of the protein produced by expression of PTEN. Based only on this information, do you predict that this protein promotes or inhibits cell division? Explain your reasoning.
PTEN inhibits cell division.
PTEN is a tumor suppressor that dephosphorylates PIP₃ to PIP₂, down-regulating the PI3K/AKT pro-growth/survival pathway, so a mutation that increases PTEN activity would be expected to reduce cell proliferation. (Note: if an activating PTEN mutation is nevertheless linked to cancer, that suggests more complex, context-dependent effects — e.g., altered regulation, different isoforms, or pathway cross-talk — but based only on the activity change, PTEN acts to inhibit division.)
Why does cancer development require mutations in multiple genes?
Cancer development requires mutations in multiple genes because it takes several changes to disrupt the normal controls on cell growth, division, and death — for example, activating oncogenes, disabling tumor suppressor genes, and damaging DNA repair genes — all of which together allow cells to grow uncontrollably and avoid normal regulation.
Explain why cancer that occurs due to mutations in the genes of body cells cannot be passed on to an individual’s offspring.
Cancer caused by mutations in body (somatic) cells cannot be passed to offspring because these mutations are not present in the sperm or egg cells—only in the affected body cells of that individual.
Can you apply the terms homozygous and heterozygous to prokaryotic cells? To gametes produced by a diploid organism? Explain your answers.
You cannot apply the term homozygous and heterozygous to prokaryotic cells because they only have one chromosome. Both gametes and prokaryotic cells are haploid which means they only have one chromosome.
Fruit files have three pairs of autosomes (chromosomes with autosomal genes). For an autosomal gene, what’s the maximum number of different alleles an individual fruit fly can have? The minimum number? What about a gamete produced by the fruit fly? Are these numbers different for humans that have 22 pairs of autosomes? Why or why not?
For each autosomal gene there are only going to be two alleles per autosomal gene, one of these can be different alleles and one must be the same allele. A fruit fly can have a maximum of two alleles and a minimum of 1. A gamete will only have one allele. These numbers ae the same compared to human since they are both diploid organisms and can only have two alleles for a given gene on their autosomal chromosomes, one from each parent.
An autosomal gene important for determining fur color in rabbits has four different alleles. How many different alleles can an individual rabbit have for the fur color gene?
An autosomal gene is important for determining fur color because the difference in alleles is the reason that they change color. An individual rabbit can have one allele that is going to be related to its fur color. This gene is going to be different based off what the allele is.
Focusing on one autosomal gene, use the steps we followed for predicting genotypes to determine the possible offspring genotypes and how likely each one is for a mating between male that is homozygous for the gene, and a female that is heterozygous for the gene. (Use any letter you want for the gene).
A homozygous male(AA) and a heterozygous female (Aa) ill have offspring that are 50% AA and 50% Aa.
A disease is determined by a single autosomal gene (i.e. the combination of alleles you have for that one gene determines whether or not you have the disease). If you are BB or Bb you have the disease, and if you are bb you do not. An individual that is heterozygous for the disease gene and an individual that does not have the disease mate. What is the probability that their offspring will have the disease? If their first offspring has the disease, does that tell you anything about the probability a second offspring will have the disease? Explain your answer.
The heterozygous parent is Bb and the healthy parent is bb, so their offspring have a 50% chance(1/2) of having the disease(Bb) and a 50% chance of not having it (bb); the first chld’s outcome doesn’t change the probability for the second child because each offpsring’s genetic are independent.
You’re studying the inheritance of two autosomal genes that each have two alleles (D or d, and Q or q). You notice that the gametes produced by an individual that’s DdQq are virtually all DQ or dq; the presence of Dq and dQ gametes is very rare. Propose a hypothesis to explain this result.
The D and Q genes are very close together on the same chromosome, so theya re usually inherited together, making DQ and dq gametes common and Dq and dQ rare.
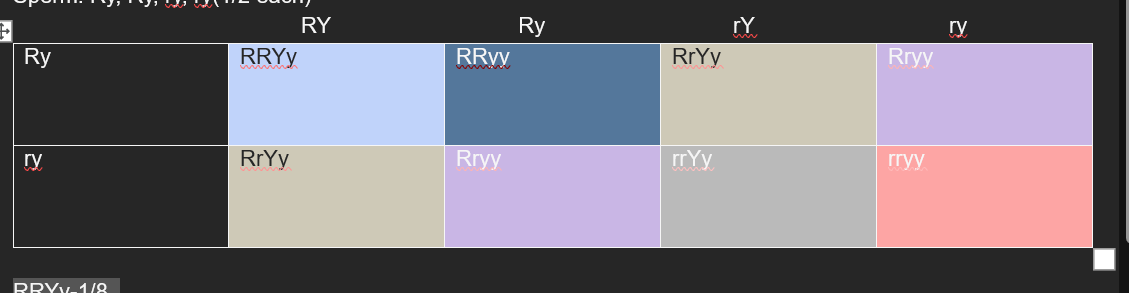
• What are the possible offspring genotypes for parents that are RrYy and Rryy? (Solution to this follow-up question is posted at the end of the slides PDF.)
RRYy-1/8
RRyy-1/8
RrYy-1/8-1/4
Rryy-2/8-1/4
rrYy-1/4
rryy-1/4
Which of the following characteristics must be common to all cells that undergo cellular division?
A. Cell gets bigger and produces more cellular molecules
B. All DNA in a cell is copied before dividing
C. The copies of DNA produced by DNA replication must be distributed to each of the new cells
D. All of the above
D. All of the above
Prokaryotic cells
A. are always haploid.
B. are always diploid.
C. can be either haploid or diploid.
A. are always haploid.
A diploid cell has four pairs of homologous chromosomes. Therefore it has a total of _______ individual chromosomes, and after DNA replication there will be _______ pairsof
sister chromatids. A. four; four
B. four; eight
C. eight; eight
D. eight; sixteen
C. eight; eight
Homologous chromosomes _______ present in a diploid cell prior to DNA replication. Sister chromatids _______ present in a diploid cell prior to DNA replication.
A. are; are
B. are; are not
C. are not; are
D. are not; are not
B. are; are not
A cell has three pairs of homologous chromosomes. If spindle fibers from the same side of the cell attach to both sister chromatids of one of the replicated chromosomes during mitosis, how many individual chromosomes will each daughter cell have?
A. one would have 12, and the other wouldn’t have any
B. one would have 7, and the other would have 6
C. one would have 7, and the other would have 5
D. each daughter cell would have 6
C. one would have 7, and the other would have 5
The process of meiosis is important for
A. sexual reproduction.
B. both sexual and asexual reproduction.
C. growth and repair.
D. sexual reproduction, growth, and repair
A. sexual reproduction.
All of the events below occur in meiosis.
Which one does NOT occur in mitosis?
A. chromosomes condense
B. spindles of cytoskeleton fibers form
C. nuclear envelope breaks down
D. homologous chromosomes pair up
D. homologous chromosomes pair up
In mitosis
A. homologous chromosomes are separated into different daughter cells.
B. sister chromatids are separated into different daughter cells.
C. both homologous chromosomes and sister chromatids are separated into different daughter cells.
D. neither homologous chromosomes nor sister chromatids are separated into different daughter cells
B. sister chromatids are separated into different daughter cells.
Which of the following describes the genetic
information in all the cells in your body?
A. They all have the same genes and one copy of each gene.
B. They all have the same genes and two copies of each gene.
C. They all have the same genes, but some have two copies of each gene and some have one copy.
D. They have different genes, but they all have two copies of each gene present in the cell.
E. They have different genes and some have two copies of a gene and some have one copy.
C. They all have the same genes, but some have two copies of each gene and some have one copy.
Which of these sources of genetic variation, if any, also apply to organisms that reproduce asexually(e.g. prokaryotes; single-celled and some
multicellular eukaryotes)?
A. mutations
B. crossing over
C. independent orientation of chromosomes
D. All of the above
E. None of the above
A. mutations
Similarities & Differences in Mitosis and Meiosis
SIMILARITIES
process of cell division for eukaryotic cells only (not prokaryotic cells) used for reproduction
DNA replicated once (and only once) before process starts
nuclear envelope breaks down and new nuclear envelopes form in resulting cells
sister chromatids get attached to spindle fibers that come from opposite sides of a cell
chromosomes get lined up in the middle of the cell by spindle fibers
sister chromatids are distributed to different cells
cytokinesis splits a cell into two cells
carefully controlled (we discussed this in the context of mitosis, but it applies to division of all cells)
DIFFERENCES
Mitosis
- purposes: repair and replacement of body cells (multicellular organisms only), asexual reproduction (single-cell and some multicellular organisms)
- initial (parent) cell can be haploid OR diploid
- homologous chromosomes do NOT pair up and are NOT distributed to different cells
- generates two genetically identical cells (cytokinesis occurs once)
Meiosis
- purpose = production of haploid gametes for sexual reproduction
- initial (parent) cell MUST be diploid
- homologous chromosomes pair up and are distributed to different cells
- generates four genetically unique cells (cytokinesis occurs twice)
A diploid organism has three different chromosomes (#1, 2, and 3). The organism inherited the exact same combination of
chromosome #2 alleles from both of its parents (i.e. the two
chromosome #2’s have the same alleles for all genes). When gametes are formed in the individual, can independent orientation generate new genetic variation?
A. Yes
B. No
A. Yes
Focusing on autosomal genes, body cells of
diploid organisms have ____ allele(s) for a
gene, and each of their gametes has ____
allele(s) for the gene.
A. one; one
B. one; two
C. two; one
D. two; two
C. two; one
How many DIFFERENT genotypes (for this gene) are possible in gametes formed by meiosis? (GGBb)
A. one
B. two
C. four
D. eight
B. two
What are the genotype probabilities for offspring generated by the parents shown below? One is Homozygous dominant and one is heterzygous
A. 1/4 QQ, 1/2 Qq, and 1/4 qq
B. 1/3 QQ, 1/3 Qq, and 1/3 qq
C. 1/2 QQ and 1/2 Qq
D. 3/4 QQ and 1/4 qq
C. 1/2 QQ and 1/2 Qq
A Punnett square to determine the possible
offspring genotypes will have _______ rows and _______ columns.
A. two; two
B. four; four
C. eight; eight
D. sixteen; sixteen
B. four; four
How many DIFFERENT gamete genotypes are
possible for an individual that is rrYyEe? (The
three genes are on different chromosomes.)
A. three
B. four
C. six
D. twelve
B. four
Pea plant flower color is determined by a single gene. If a pea plant is true-breeding for white
flowers, you know that
A. it is homozygous for the white flower color allele.
B. it is heterozygous for the flower color gene.
A. it is homozygous for the white flower color allele.
Based on what you know about true-breeding plants
and predicting offspring genotypes,
A. the offspring of this cross will all be homozygous for the round allele.
B. the offspring of this cross will all be homozygous for the
wrinkled allele.
C. the offspring of this cross will all be heterozygous for the pea shape gene.
D. some offspring of this cross will be homozygous for the round allele, and others will be homozygous for the wrinkled allele.
C. the offspring of this cross will all be heterozygous for the pea shape gene.
The possible gamete genotypes for an individual that is RrYy are
A. Rr and Yy. '
B. R, r, Y, and y.
C. RY, Ry, rY, and ry.
C. RY, Ry, rY, and ry.
Why is it important to look at lots of offspring (a large sample) when assessing patterns of inheritance?
This is important because looking at only a few offspring can cause you to miss things compared to when you look at alarge sample. There is things that are going to happening in some offspring while they’re not happening in the others. An example of this is a lot of cells are in interphase, its 90% of the time that a cell is in interphase doing others things, but 10% of the time its in mitosis and its replicating. If you’re only looking at a few cells then you are going to miss the few cells that are replicating.
Explain why you know a plant that’s true-breeding for a particular trait is homozygous for the gene associated with that trait.
I know that a plant that’s true-breeding for a particular trait is going to create also homozygous alleles because true-breeding always creates the SAME traits as the parents, it cannot create a different trait.
You discover a new species of berry plant and observe that some of the plants have pink berries and others have yellow berries. Assuming that berry color is a Mendelian trait in this species, design an experiment to determine whether yellow berries are dominant or recessive compared to pink berries.
If you cross a pink-berried plant with a yellow-berried plant you will be able to tell which of the offspring are dominant based on what color the offspring are. If they are yellow then the yellow is dominant, if the pink then the pink is dominant.
Round peas are dominant to wrinkled peas and yellow pea color is dominant to green pea color in a species of pea plant. Using R as the allele for round peas, r as the allele for wrinkled peas, Y as the allele for yellow peas, and y as the allele for green peas, figure out the possible offspring phenotypes and their probabilities from a cross between plants that are RrYy and Rryy.
RrYy gametes- RY, Ry, rY, ry
Rryy gametes-Ry, ry
| RY | Ry | rY | ry |
Ry | RRYy | RRyy | RrYy | Rryy |
ry | RrYy | Rryy | rrYy | rryy |
RRYy-1/8
RRyy-1/8
RrYy-2/8
Rryy-2/8
rrYy-1/8
rryy-1/8
In pea plants, purple flower color is dominant to white flowers (no color). You’re given a plant with purple flowers, but don’t know whether it’s heterozygous or homozygous for the flower color gene. If you crossed the purple plant with a true-breeding white flowered plant, would you be able to determine the genotype of the purple-flowered plant from the F1 offspring’s phenotype(s)? If so, what are the expected offspring phenotype probabilities if the purple flowered plant was heterozygous for the flower color gene? Homozygous?
Yes, you can determine the genotype by crossing with a white-flowered plant.
If the purple plant is heterozygous(Pp), offspring 50%, white 50%
If the purple is homozygous(PP)-100% purple
Explain why the gametes produced in males that have X chromosomes always have the same alleles for all X-linked genes. Explain why gametes produced in females can have different alleles for X-linked genes.
The gametes produced in males that have X chromosomes always have the same alleles for all x-linked genes because males only have one x-linked chromosome since the other sex-linked chromosome is a Y chromosome. Gametes produced in females can have different alleles for x-linked genes because there are two x chromosomes meaning that there can be different alleles.
X-linked recessive diseases are due to a recessive allele for a particular X chromosome gene in humans. Explain why X-linked recessive diseases affect more males than females.
X-linked recessive disease affects males more than females because males only have a 50/50 of their genes and allele differences. But for women there are two X chromosomes meaning that they can also be heterozygous which gives them more of a chance to be dominant for that gene.
In fruit flies, red eyes is dominant and white eyes is recessive. A red-eyed female fruit fly mates with a white-eyed male. If some of their male offspring have white eyes, what (if anything) do you know about the genotype of the female fly?
If some of their offspring have white eyes we are able to conclude that the female fly was heterozygous meaning they do have one recessive gene but it is paired with a dominant gene which shadows over the recessive gene.
Can a male be heterozygous for an X-linked gene? Why or why not?
A male cannot be heterozygous for an X-linked gene because they only have one x-linked gene compared to females who two so they can be heterozygous for genes.
Sexual Reproduction
fusion of egg and sperm, genetically unique offspring, multicellular offspring
asexual reproduction
one parent produces genetically identical offspring, prokaryotes & eukaryotes, single & multicellular organisms
Diploid
two sets of chromosomes
haploid
one set of chromosomes
allele
a specific version of a gene that determines a trait
Homologous chromosomes
a pair of chromosomes with the same set of genes
sister chromatids
duplicate copies of an individual chromosome generated by DNA replication
non-sister chromatids
chromatids of a homologues chromosome pai that are NOT sisters
independent orientation
different combinations of alleles for genes on different chromosomes than were present in egg & sperm cells that formed the individual parents
crossing over
different combinations of alleles for genes on the same chromosome
Genetic Variation
differences in the info(DNA) required to create and maintain cells
random fertilization
which specific egg is fertilized by which specific sperm is completely random
character
physical or functional feature that can be inherited(influenced by genes)
trait
variants of a character(influenced by alleles)
Genotype
combination of alleles
Phenotype
combination of traits
Autosomal genes
genes located on chromosomes common to males & females
Homozygous
SAME alleles for a gene
Heterozygous
DIFFERENT alleles for a gene
self-fertilization
eggs fertilized by sperm from the SAME plant
true-breeding
ALL offspring of self-fertilization always have the same traits as the parent