6B - Aromatic Compounds and Amines
1/32
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
33 Terms
What is the formula of benzene?
C6H6
What is the structure of benzene?
Six carbon atoms joined together in a flat ring
It’s unpaired electron is locating in a p-orbital that sticks out above and below the plane of the ring
Delocalised electrons form a ring
What is the length of bonds between a benzene ring?
In between a single and double bond
Explain the stability of benzene in comparison to other compounds
Benzene is more stable as had a lower enthalpy change of hydrogenation than expected
How are aromatic compounds named?
If benzene is the main functional group the suffix is -benzene
If not -phenyl or -phen is used
Which molecules use phenyl to name?
Amines, alcohols, ketones, alkenes
Why does abenzene ring attract electrophiles?
Benzene ring is a region of high electron density
What mechanism occurs with a benzene ring?
Electrophilic substitution
What is nitration?
When benzene is warmed with conc nitric acid and sulfuric acid nitrobenzene is produced
What is the equation for the formation of the nitronium ion?
HNO3 + H2SO4 → HSO4- + NO2+ + H2O
What temp does the nitration need to be kept below for mononitration?
55c
What can nitro compounds be used for?
Manufacture of dyes and pharmaceuticals or explosives
What is friendel-crafts acyl acylation?
An acyl group is added to a benzene ring
What are the products of f-c a?
HCl and phenylketone
What are conditions are needed for f-c acylation?
Heated under reflux in non-aq solvent with AlCl3
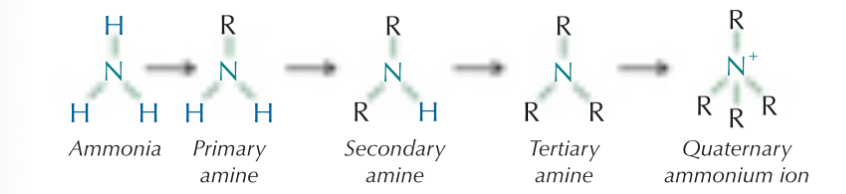
What are amines?
One or more hydrogens on ammonia is replaced with an organic group
What are the different stages of amines?
What do amines smell like?
Fishy
What are quarternary ammonium salts?
Quarternary ammonium ions form complexes with negative ions
How are amines named?
Suffix is -amine
di- tri- tetra- (secondary, tertiary…)
What are cationic surfactants?
Quarternary ammonium salts with one long hydrocarbon that are partly soluble and partly insoluble
Why are xationic surfactants useful as detergents?
Hydrocarbon chain binds to non-polar substance, polar head group is soluble in water
What are cationic surfactants used as?
Fabric conditions, hair products, detergents
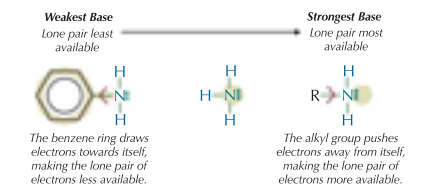
Why do amines act as weak bases?
They accept protons as there’s a lone pair of electrons on the nitrogen that can form a co-ordinate bond
What does the strength of the base rely on?
How available the lone pair is
What are amides?
-CONH2 (derivatives of carboxylic acids)
Why do amides behave differently than amines?
The carbonyl group pulls electrons away from the NH2 group
What are N-substituted amides?
One hydrogen attached has been substituted with an alkyl group (add N- to the start)
How are amines formed from halogenoalkanes?
Nucleophillic substitution (further substitutions can take place until a quarternary ammonium salt is formed)
How are amines formed from nitriles?
Reduced an amine using a strong reducing agents (LiAlH4) in dry ether with a dilute acid
Why is LiAlH4 not used in the lab and what is used instead?
Too expensive so platinum or nickel catalyst is used
In what mechanism are amines used as nucleophiles?
Nucleophillic addition-elimination
How are aromatic amines formed?
Reducing a nitro compound using tin and conc HCl under reflux
Then add sodium hydroxide