1- Atomic Structure
1/54
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
55 Terms
How do properties of isotopes differ?
Chemical properties are identical (same electron number, same electron configuration)
Different physical properties due to differences in mass (density, radioactivity, melting point)
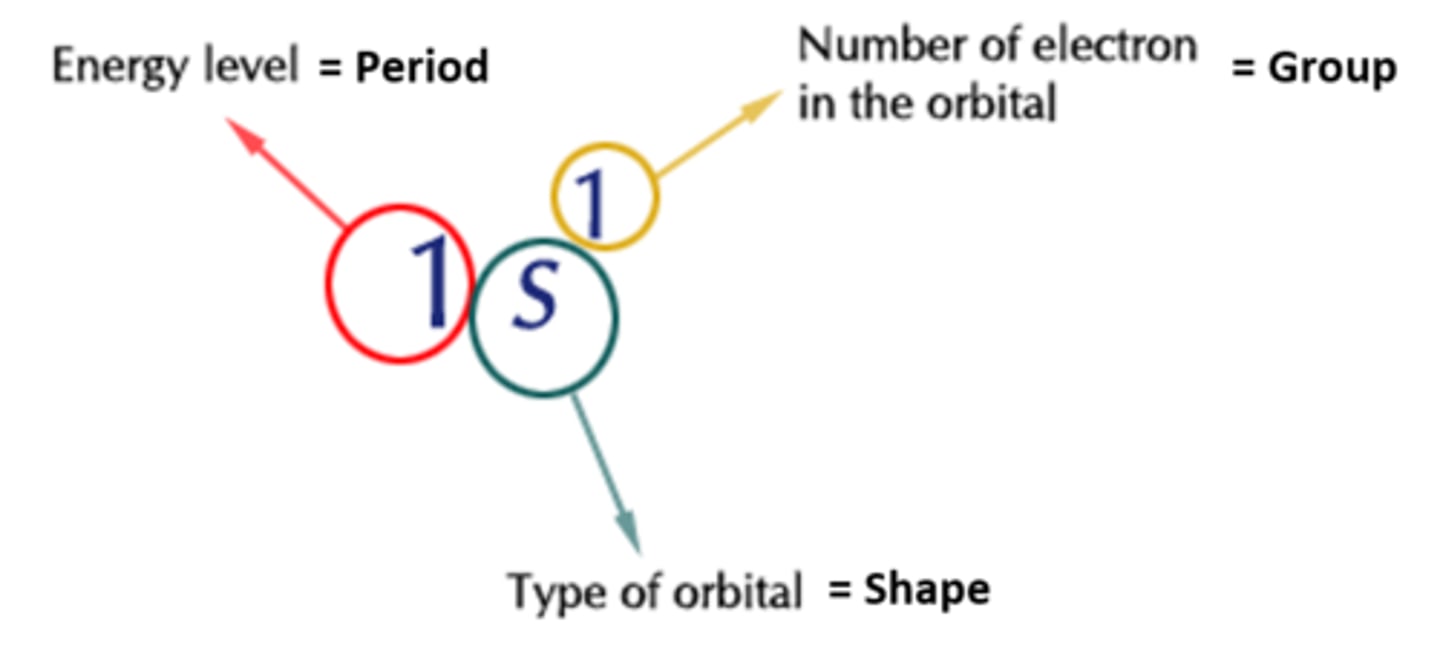
How to write electron configurations
1. The period number refers to what row the element is in
2. The letter refers to what sub orbital it is in (s,p,d,f)
3. The higher number refers to how many electrons are in that sub-orbital (how far along In the block eg carbon 2)
Start from the top of the periodic table writing down the sub orbital values as the element fills each one until the element itself is reached. Use the steps above to figure out the desired electron configuration
What is the mass number? What is the relative atomic mass?
Mass number - how many protons and neutrons there are in one atom
Relative atomic mass - the weighted abundance of an entire sample
How many electrons can S, P F and D hold?
S=2
P=6
D=10
F-14
Where are the S, D, F and P blocks found in the periodic table?
S block - Group 1/2 (as the outer shell electrons occupy the s orbital)
D block - Transition metals (outer shell electrons occupy the D orbital)
P - Groups 3-7
F- 2 bottom rows
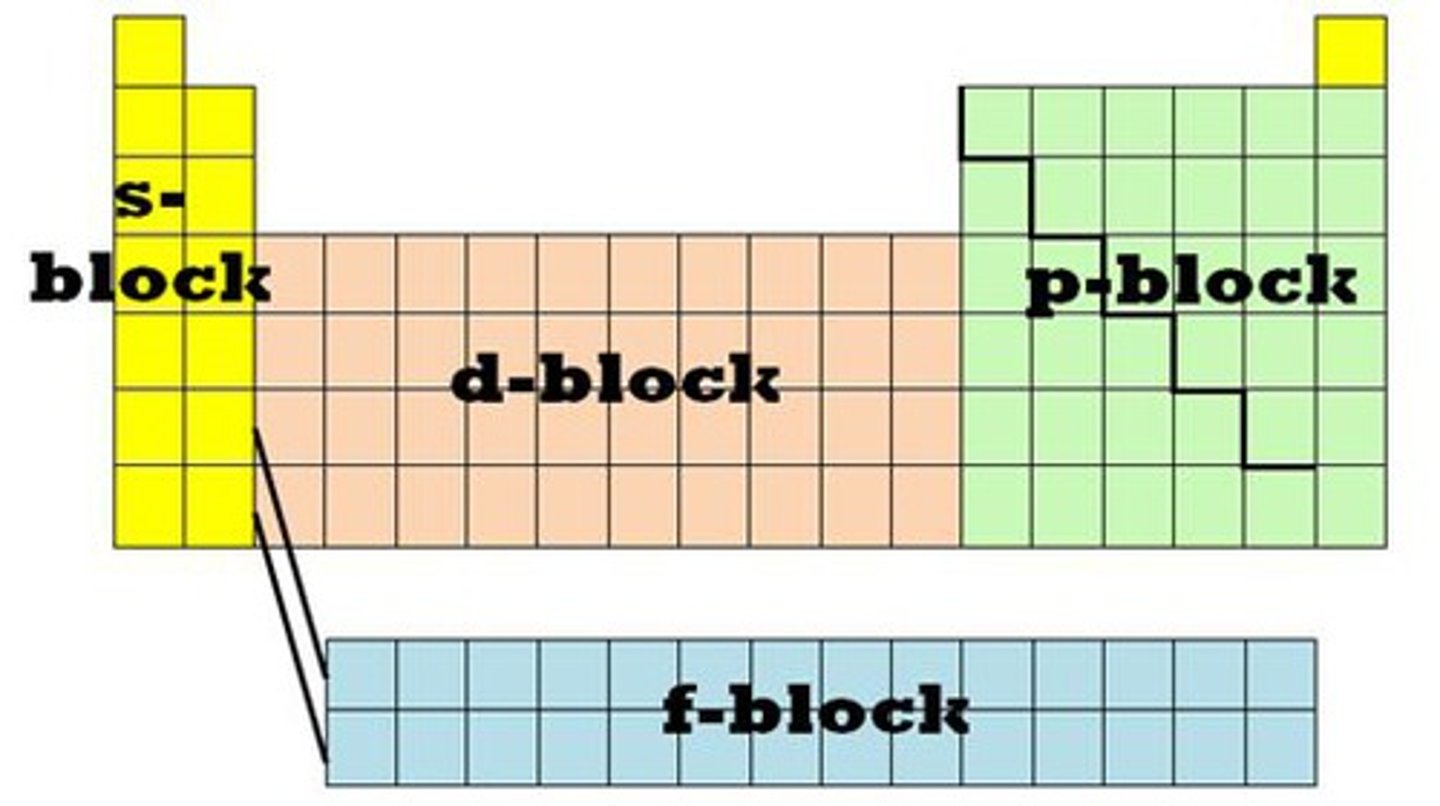
Explain where each sub group of S,D,P,F are
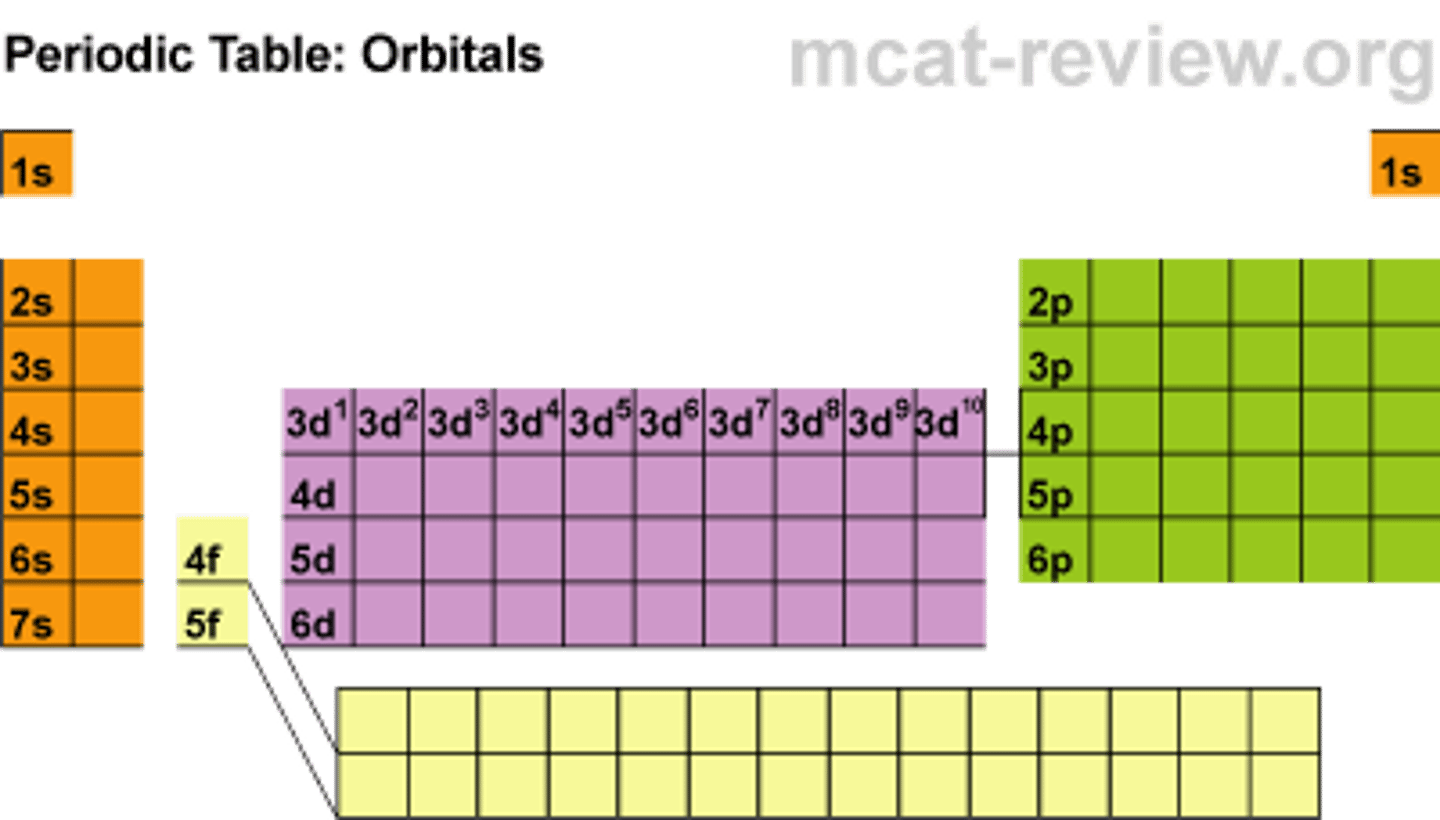
Rules when writing the electron configurations for Chromium and Copper.
You would expect to write '4s2, 3d4' when writing the configuration for chromium. Instead one electron jumps from the 4s2 to the 3d4'. This creates '4s1, 3d5'. For chronium, this occurs to allow it to have a more stable half sub shell.
This rule also applies for copper, to allow it to have a full d sub shell (10e-) so it to can be more stable. NOTE: In some cases, you can use the last noble gas (Ar) to represent the electron configuration so far, then continue. For example Se: [Ar] 4s2, 3d10, 4p4
Rule when writing the electron configurations for ionising transition metals
When we ionise transition metals (d block elements), we loose electrons from the 4s orbital first instead of the 3d orbital. For example Fe -> Fe3+. The Fe configuration is ...4s2, 3d6. The three electrons are lost, but first from the 4s2. Fe 4s2,3d6 --> Fe3+ 3d5
P electron configuration
1s2,2s2,2p6,3s2,3p3
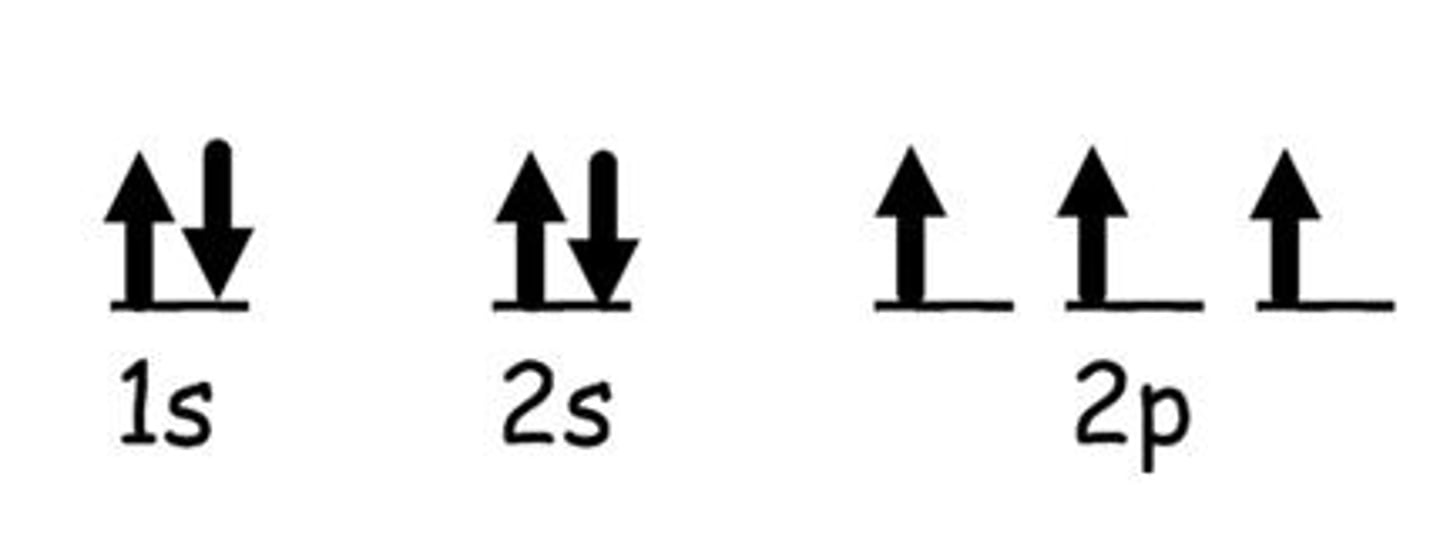
What do the arrows represent in electron configurations?
Each of the boxes represents one orbital. Each arrow represents one electron. The up and down arrow represents the electrons spinning in opposite directions. Two electrons can only occupy the same orbital if they have opposite spin
What did Neil bohrs model suggest?
-Electrons orbit the nucleus in shells.
-Each shell has a fixed energy
-When an electron moves between shells electromagnetic radiation is emitted or absorbed.
-Because the energy of the shell is fixed, the radiation will have a fixed frequency.
Cr3+ Electron configuration
[Ar] 3d3
Timeline of the atomic models
Dalton > JJ Thomson > Rutherford > Bohr
What did Dalton suggest in his version of the atom?
Described atoms as solid spheres, and said that different spheres made up the different elements
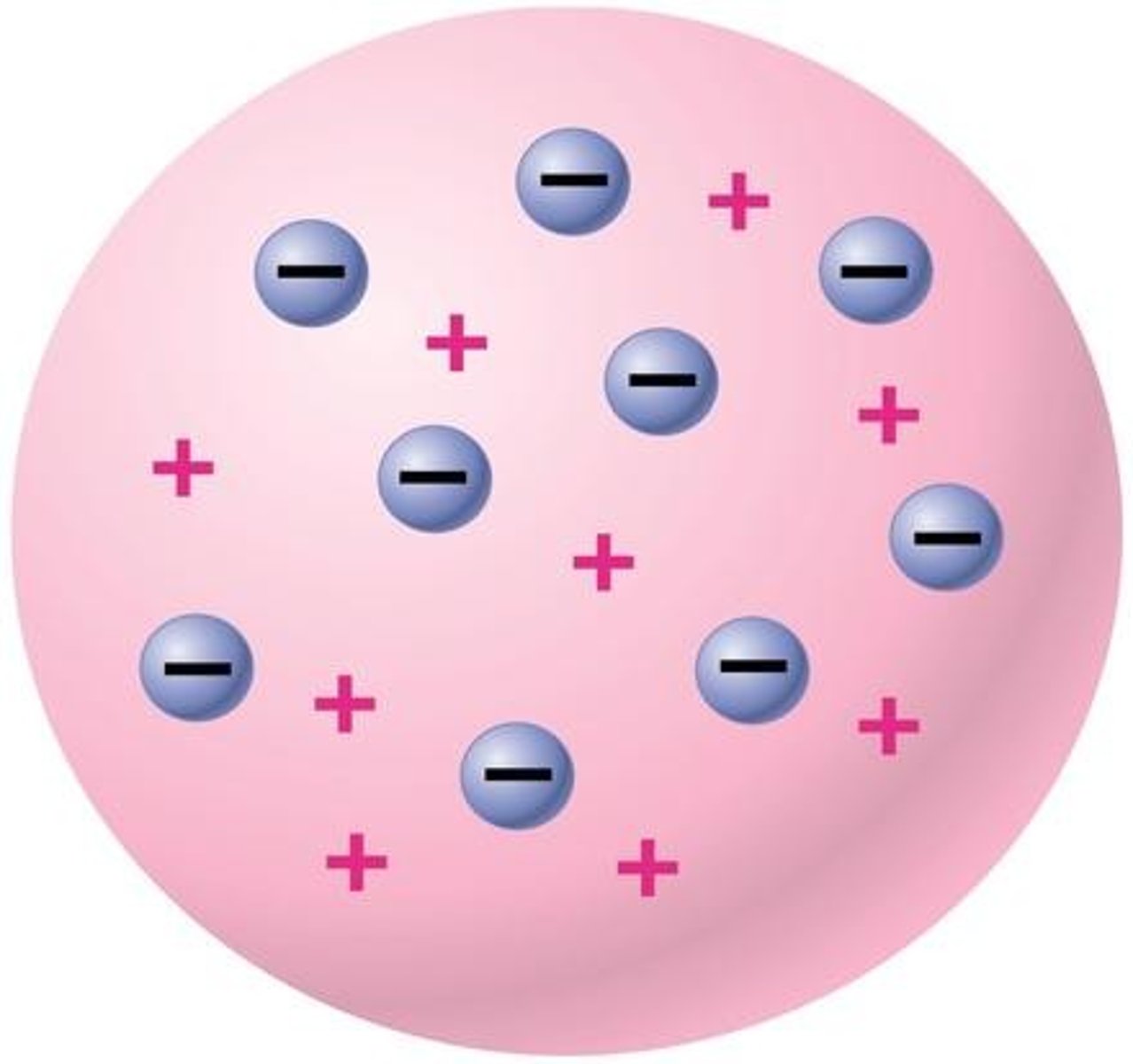
What did JJ Thomson suggest in his version of the atom? How did this come about?
Concluded that an atom must contain even smaller, negatively charged particles - electrons. These electrons were embedded in the positively charged mass. He discovered this using a cathode ray tube.
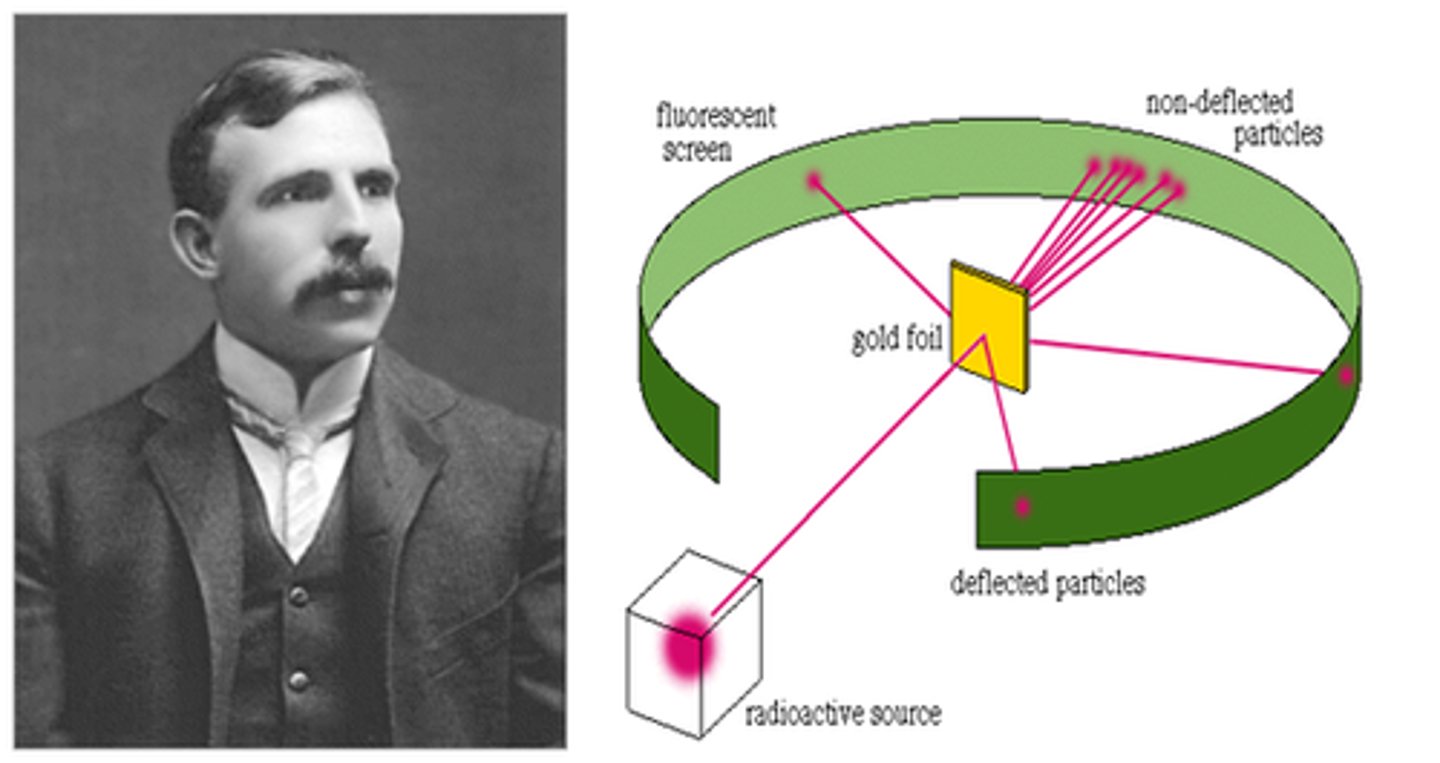
What did Ernest Rutherford suggest in his version of the atom? Explain his experiment
That the atom was mostly empty space, with nearly all of its mass concentrated in a tiny nucleus. He discovered this using his 'alpha scattering' experiment. This involved firing positively charged alpha particles at a tiny sheet of gold foil. Unexpectedly, most of the atoms passed through.
What is mass spectrometry?
Analytical technique used to identify different isotopes and find the overall relative atomic mass of an element. (mass to charge ratio)
Explain the ionisation stage of mass spectrometry
A sample of an element is vaporised and injected into the mass spectrometer.
Hard ionisation - due to electron impact, fire high energy electrons using electron guns, electrons are knocked off, molecules are now +ions. (No mass change as a mass number = proton + neutron). Causes sample to FRAGMENT
Soft ionisation (ELECTROSPRAY) - Dissolve the sample in a polar volatile solvent, inject through a needle attached to a high voltage. The high voltage rips a proton off the solvent and attaches it to the sample molecules. The sample molecule are now positively charged. (Mass change as a proton has been gained). Sample does NOT FRAGMENT.
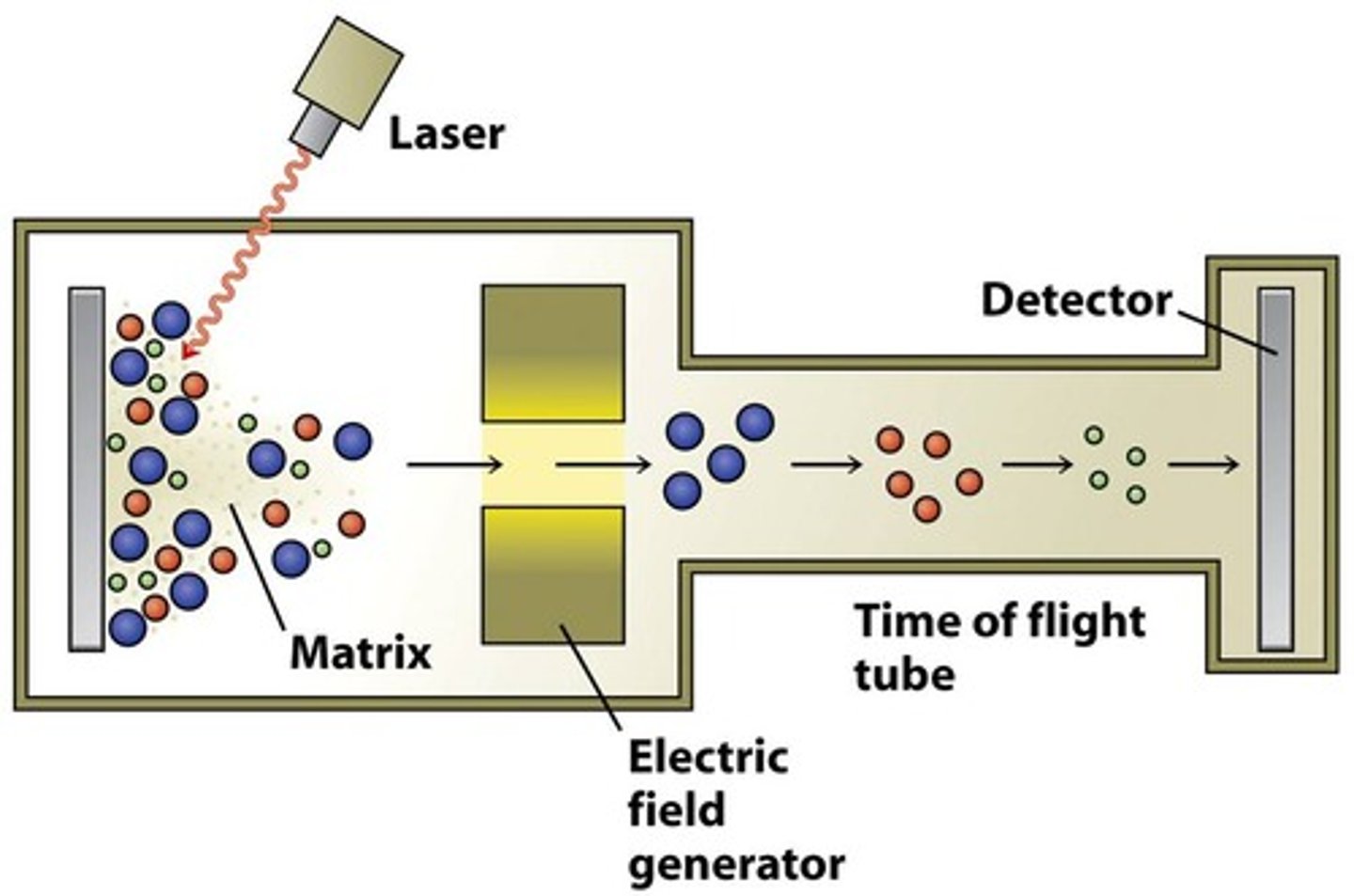
Explain the acceleration phase of mass spectrometry
Positive ions are accelerated by an electric field and by the attraction to the negative plate. Molecules accelerated all have the same kinetic energy therefor, lighter ions will travel faster than heavier ions.
KE = 1/2 mv2
Electrospray equation
M + H+ -> MH+
(The solvent acts as a source of protons to facilitate the ionisation)
Explain the ion drift stage of mass spectrometry
The region of a spectrometer is where the ions are separated. The 1+ ions will pass through a hole in the negatively charged plate and move into the flight tube. The time of flight of each 1+ ion depends on its velocity.
Explain the detection phase of mass spectrometry
The ions hits the negatively charged plate.
This causes a current and the size of this current gives a measure of the number of molecule hitting the plate.
This gives the abundance of the molecule.
current size = abundance
How to calculate TOF
KE = mv^2
Velocity is also calculated as v = s/t.
If we substitute this into the equation KE = mv^2 , we get the equation shown in the photo attached
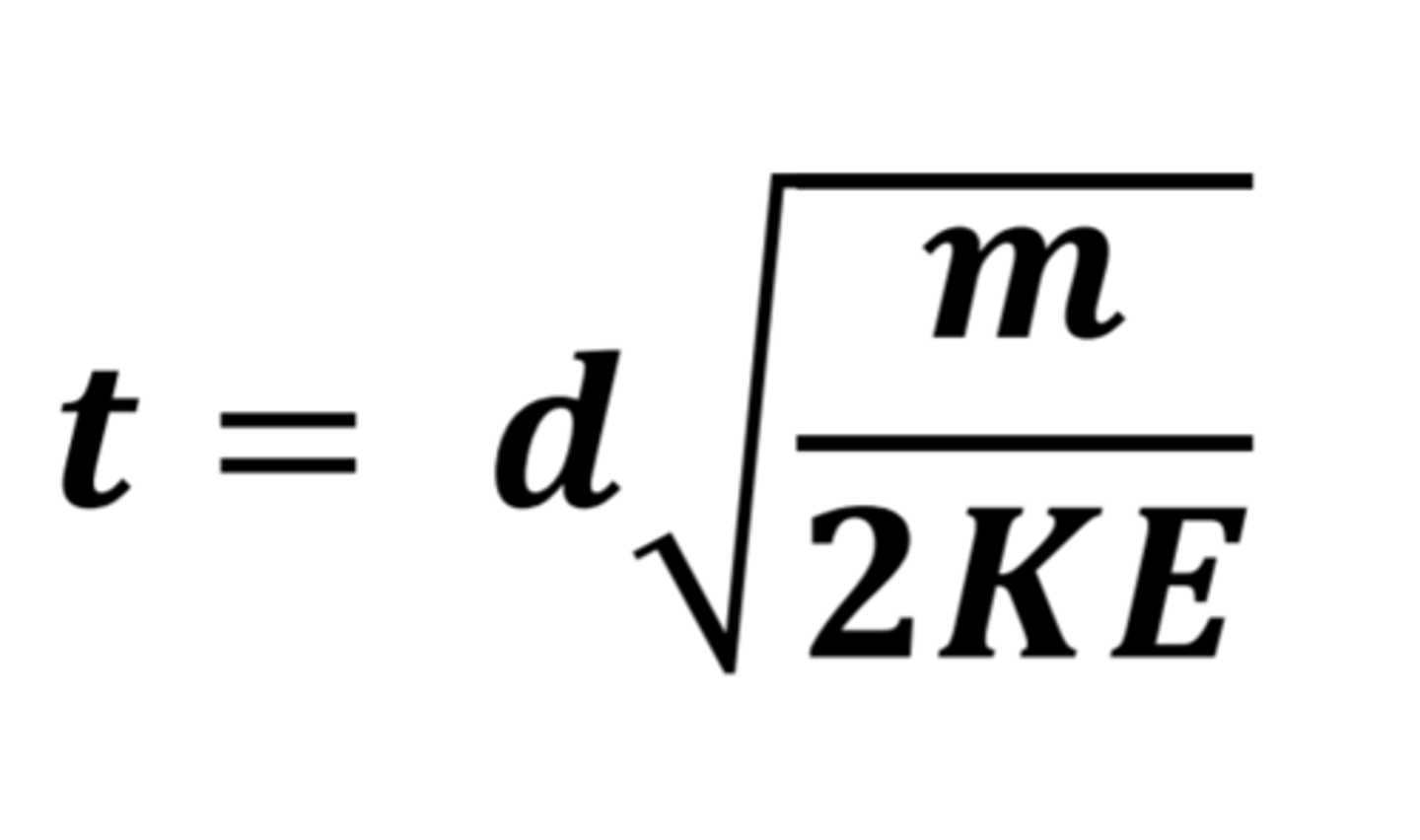
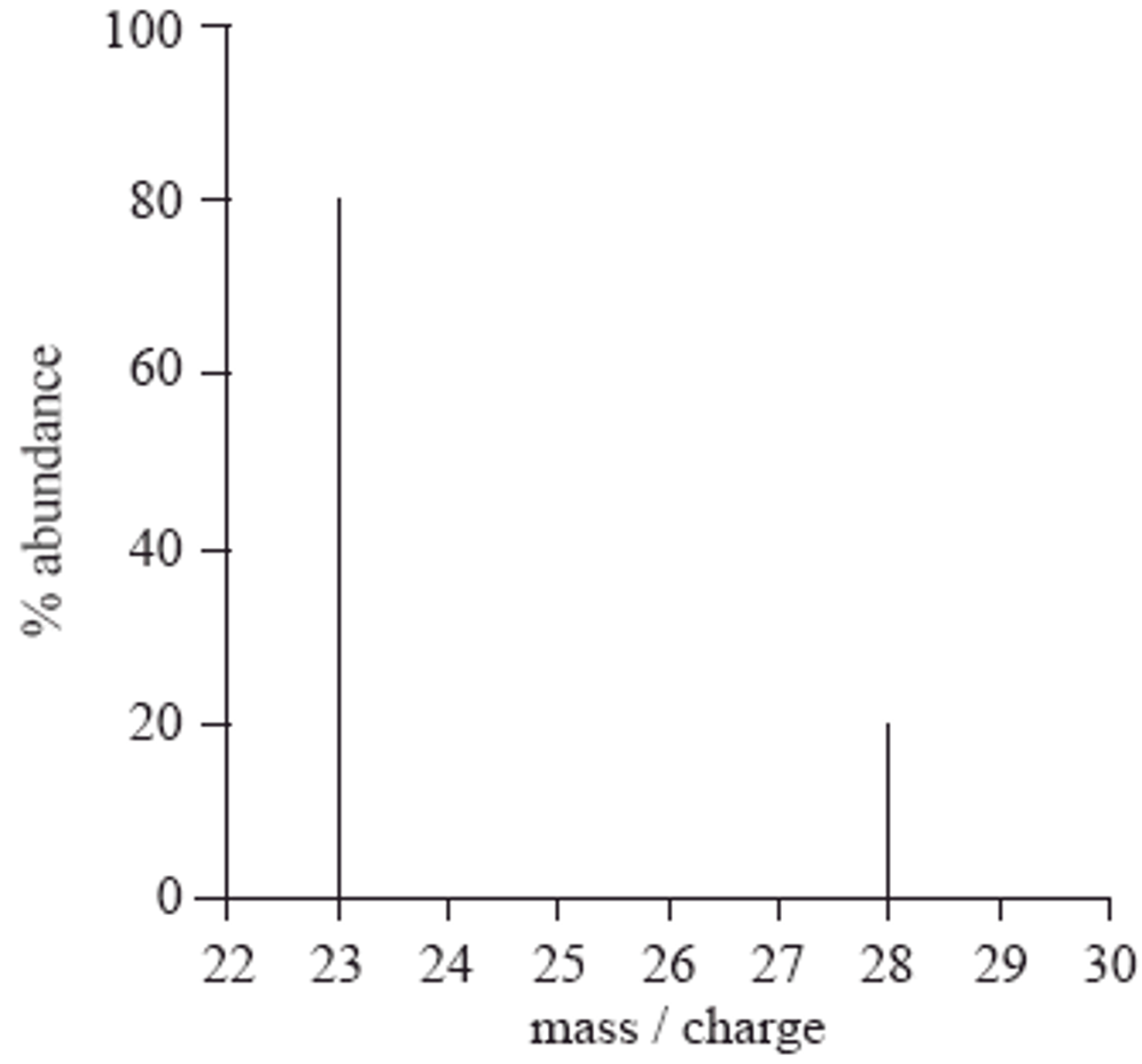
How to interpret a mass spectrometry graph
Work out the relative atomic mass.
In the graph for example: (20%x28) + (80%x23) /100. The base peak is the tallest line as it is the most abundant ion in that atom.
What is the difference between mass and relative atomic mass?
Relative atomic mass considers the weighted average of all isotopes of the element, whereas mass number considers the number of protons and neutrons
Define relative atomic mass
Average mass of an atom/ 1/12th mass of carbon-12
How does. M2+ ion occur in mass spec
It is possible for 2 electrons to be removed, creating a M2+ ion.
Since ions are sorted according to their m/z, they ac like an ion with halve the mass of a + ion. They accelerate more
What type of samples is hard/soft ionisation for?s
Hard (electron impact) - smaller molecules which are unlikely to fragment
Soft (electrospray) - larger organic molecules as electron impact would cause fragmentation, prefer to stay intact
Describe how ions are formed in a mass spectrometer (2)
-A high voltage is applied to the sample in a polar solvent
-The sample molecule M gains a proton forming MH+
Write an equation, including state symbols, to show how an atom of Vanadium is ionised by electron impact
V(g) +e- > V+(g) + 2e-
An atom of 58Ni is ionised using electrospray ionisation. What will be the mass of the ion created?
59
A sample of chlorine was analysed in a time of flight mass spectrometer and found to contain two isotopes 35Cl and 37Cl.
After electron impact ionisation, all of the atoms were accelerated to the same kinetic energy and then travelled through a flight tube that was 2m long.
The 35Cl ions took 7.5x10^-4s to travel through the flight tube.
a) Calculate the mass, in kg, of one ion 35Cl
b) Calculate the time taken for the 37Cl ions to travel through the same flight tube
a) use avagadros constant to work out how much an ion weighs in grams 35/6.022x10^23 = 5.81202x10^-23
convert into kg 5.181202x10^-23 / 1000 = 5.81202x10^-26
b) velocity = distance / time
2/7.5x10^-4 = 2.67x10^3
KE = 1/2mv^2
KE 35Cl = KE 37Cl KE = 1/2 mCl35 v^2 = 1/2 mCl37v^2
(1/2s cancel out) mCl35 v^2 = mCl27 v^2
Rearrange mCl35v^2 / mCl37 = v^2 v^2 of 37 Cl = (2.67x10^3)^2 x 35/37
= 6.743554054x10^6
= square root your answer
= 2.596835392x10^-3
Write an equation for the reaction that occur when a tellurium ion hits the detector
Te+ + e- >>> Te
The mass spectrum of tellurium also has a small peak at m/z = 64. Explain the existence of this peak
2+ ion formed / 2 electrons removed from 128 Te
Explain why it is necessary to ionise molecules when measuring their mass in a TOF mass spectrometer
Ions, not molecules, will interact and be accelerated by an electric field. Only ions create a current when hitting the detector.
A sample of element R contains isotopes with mass number 206,207,208 in a 1:1:2 ratio of abundance. Calculate the relative atomic mass of R. Give you answer to one DP
Convert ratios into percentages.
1+1+2 = 4
1/4 = 25%
1/4 =25%
2/4 = 50%
(0.25x206)+(0.25+207)+(0.5x208) = 207.3
A sample of element Boron has a relative atomic mass of 10.8. In this sample, boron exists as 2 isotopes 10B and 11B. Calculate the percentage abundance of 10B
10x/100 + 11(100-x)/100 =10.8
10x+1100 -11x+ 1080
x=1100-1080 = 20%
What is ionisation?
Process which adds or removes electrons from an atom/molecule. The process is endothermic, as energy is required to break the forces of attraction between the nucleus and outer electrons
Explain the first ionisation energy
Energy required to remove 1 electron from each atom of an element in 1 mole of gaseous atoms, to form 1 mole of gaseous atoms with a 1+ charge
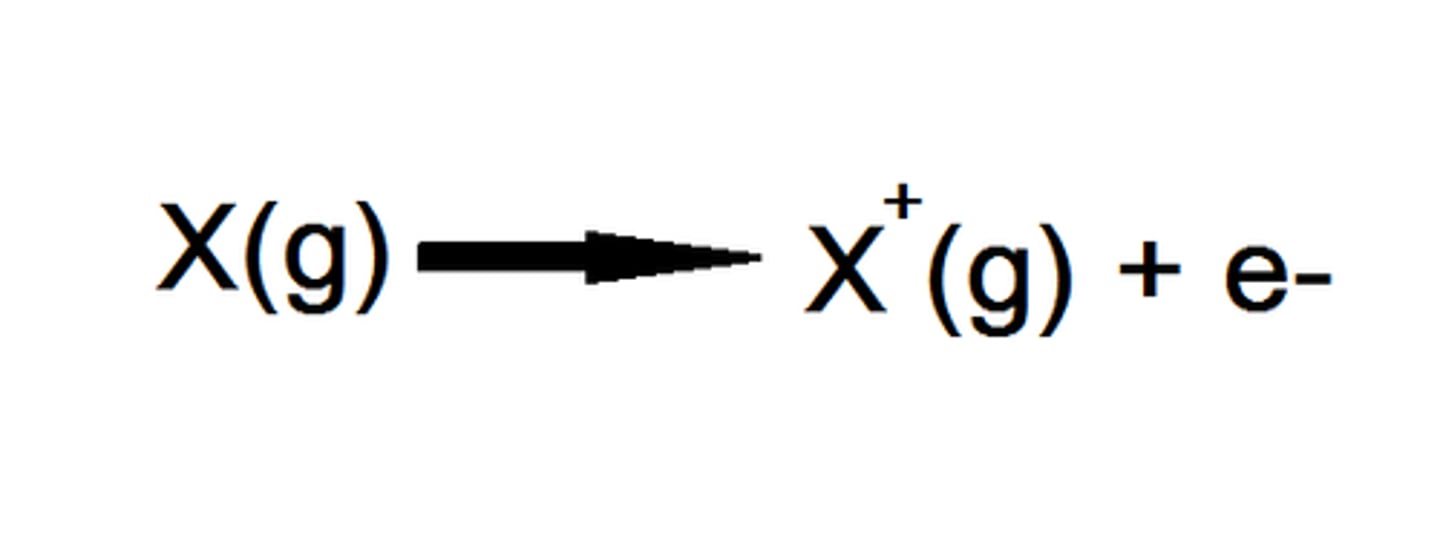
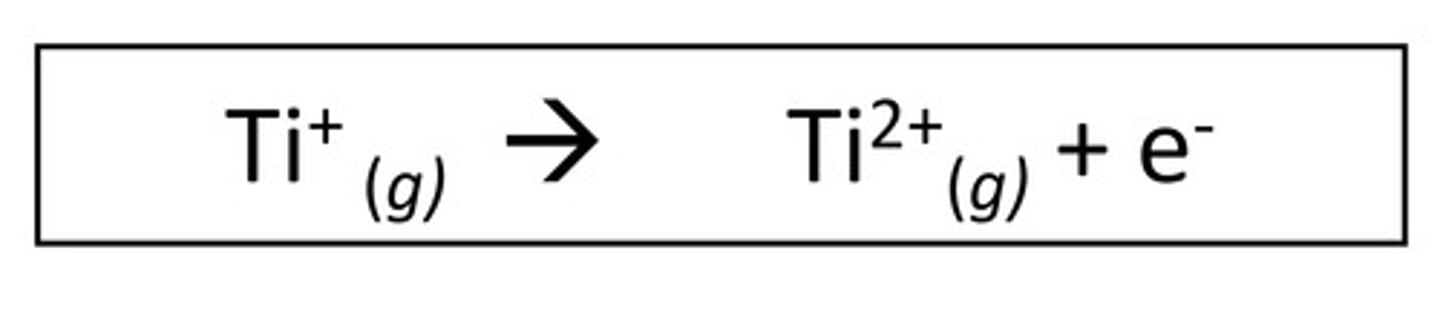
Explain the second ionisation energy
Energy needed to remove one mole o electrons from one mole of 1+ ions in their gaseous state to form one mole of 2+ ions
Explain the factors affecting ionisation energies
-Nuclear charge (the higher the atomic number of an element is, the more protons it has in its nucleus, hence the higher the nuclear charge. Increasing the ionisation energy
-Atomic radius (the higher the radius, the lower the ionisation energy. Due to the outer electrons being further from the nucleus, weaker attraction)
-Number of inner shells (the more inner shells present, the lower the ionisation energy. More shielding, weaker attractions)
Explain the oxygen ionisation energy graph
-Gradual increase (each time we remove an outer electron the remaining electrons and pulled slightly closer to the nucleus, hence a greater attraction)
-Rapid increase (oxygen has 6 electrons in its outer shell, when we remove the 7th, it. is removed from the first electron shell. Electrons experience less shielding, hence a greater attractio)>
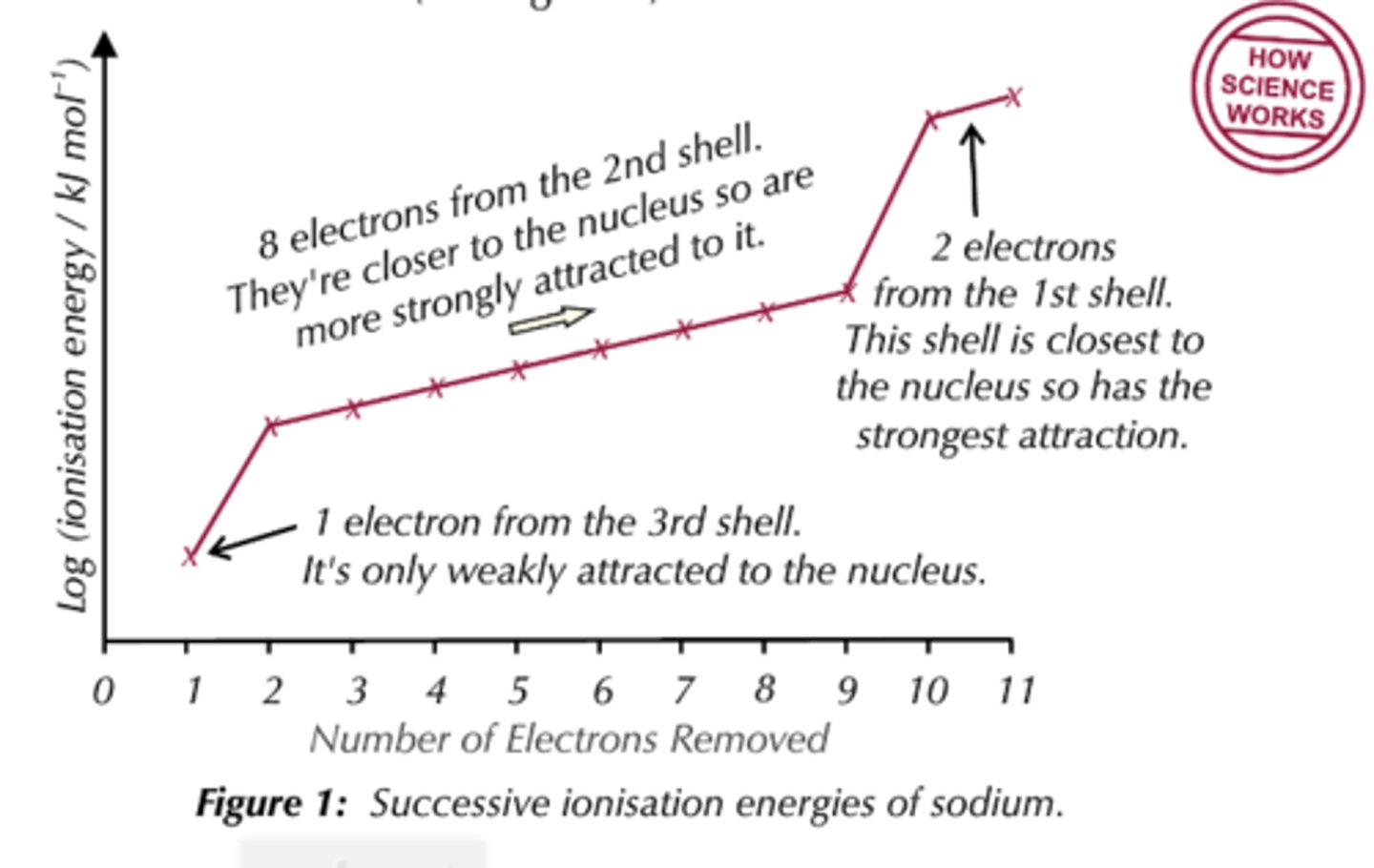
Explain why the ionisation energy decreases going down group 1
-Atomic radius increases
-More shielding
NOTE: Even though as you go down the group nuclear charge increases, the two factors above outweigh this
Why does the first ionisation energy generally increase across a period?
-Nuclear charge increases
-Atomic radius decreases (increased nuclear charge increases the attraction between nucleus and electrons resulting in decreased atomic radius).
Why does Boron have a lower first ionisation energy than beryllium?
Configuration: [He] 2s 2p1
Outer electrons are in a 2p shell, which has a higher energy than a 2s sub shell.
This means it takes less energy to remove the outer electron of Boron compared to the outer electron of Beryllium (2s subshell)
Why is the first ionisation of oxygen less than nitrogen?
Oxygen: [He] 2s2 2p4
Nitrogen: [He] 2s2 2p3
Nitrogen has 3 electrons in each orbital
Oxygen has 3 in each box, with one orbital having another electron. The two electrons repel, this takes less energy to remove one of these electrons than if the electrons were in separate orbitals.
As a result the first ionisation energy of oxygen is less than nitrogen.
How to explain ionisation energy graphs
Use electron configurations
Order of energy sub shells, highest to lowest
3p
3s
2p
2s
1s
State two features of the current model which is not shown in the Rutherford model
-Current includes protons and neutrons
-Current has electrons in different orbitals
Explain the pattern of first ionisation energies of elements from Li to Ne (6)
STAGE 1: 1st IE increases/ due to more protons/ leading to an increased nuclear charge/ no extra shielding/ strong attraction between nucleus and outer shell electrons
STAGE 2: Deviation Be to B
B has a lower IE than Be as the outer electron is in a P sub shell, higher energy that 2s
STAGE 3: Deviation N to O
O Lowe than N/ 2 electrons in 2p needed to pair/pairing causes repulsion/ less energy needed to remove electron in O
A sample of ethanedioic acid was treated with an excess of an unknown alcohol in the presence of a strong acid catalyst. The products of the reaction were separated and analysed in a time of flight (TOF) mass spectrometer. Two peaks were observed at m / z = 104 and 118.
(a) Identify the species responsible for the two peaks.
a) CH3OCOCOOH+
CH3OCOCOOCH3+
Explain why the instrument in mass spectrometry is kept under a vacuum
Prevents any ions produced from colliding with molecules in the air (path not obstructed)
Explain how an electric current is produced as the ions hit the detector
When the ions hit the plate, they are discharged by gaining electrons from the plate. This generates a movement of electrons and hence a electric field is produced
Electron impact equation
X(g) + e- -> X+(g) + 2e-
Give the full electron configuration of the element in Period 3 with the highest first ionisation energy
1s2 2s2 2p6 3s2 2p6 (argon)