Alkenes
1/67
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
68 Terms
what is an alkene?
unsaturated hydrocarbon: contains at least 1 double bonds and is not a ringed structure
alkene functional group?
C=C
alkene general formula?
CnH2n
for a molecule to be an alkane, what characteristics must it have?
be a hydrocarbon (H&C only)
not contain a ring
contain a C-C double bond: all alkenes are unsaturated
suffix for alkenes?
-ene
how do we name alkenes, what do we prioritise when naming carbon chains?
number the carbon parents chain so the C=C double bond has the lowest possible position number
how do we represent double bonds in skeletal formulas?
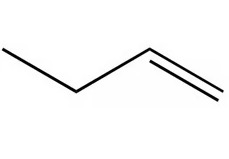
process for naming alkenes?
identify if its an alkene; add -ene
number carbons in main chain and add prefix (stem name)
if needed add a position number of the double bond and side chain position
if theres stereoisomers add E or Z
for branched alkenes, what must the parent chain contain?
a double bond
what is the main chain in alkenes?
longest chain of carbons containing a double bond
properties of alkenes?
region of high electron density around the double bond
non-polar
highly insoluble in water
van der waals only
as length of carbon chain increases, the strength of van der waals increases, so melting and boiling point increases
why does melting point and boiling point increase as the length of the carbon chain increases?
more electrons, increases the strength of van der waals as theres more opportunities for an induced dipole, so more energy is required to break the intermolecular forces
why are alkenes highly insoluble in water?
alkenes are non-polar, water is polar, so alkenes cannot dissolve
what does bonding in alkenes involve in terms of bonds and electrons?
a double covalent bond and a centre of high electron density
what conditions are required for rotation of alkenes around the axis occur?
high temperatures
or
if alkene is the right shape and size to absorb photons of visible light
why does rotation of alkenes require these restricted conditions?
rotation requires a lot of energy as pi bonds in the double bonds needs to be broken
when alkanes are rotated, what happens to the overlap of orbitals of sigma bonds?
stays the same
when alkenes are rotated, what happens to overlap of orbitals of sigma bonds and orbitals of pi bonds?
sigma: stays the same
pi: decreases to no overlap = broken pi bond
when does the pi bond reform after rotation?
after rotated a full 180 degrees
why can alkanes rotate freely but alkenes cannot?
alkanes can change their shape as they only have single, sigma bonds, alkenes have double, pi bonds which require a lot of energy and restricted conditions so they have restricted rotation around double bonds
how many degrees can double bonds rotate at high temperature or if it can absorb photons?
can rotate up to 180 degrees, but can be restricted if conditions arent met
what is a stereoisomer?
compound with the same structural and molecular formula but 2 different spatial arrangements of atoms
why are alkenes stuck in 1 arrangement or the other? (isomers)
restricted rotation around the double bond = different properties = different isomers
why can alkanes be either arrangement of isomers?
carbon atoms can rotate freely about the bond axis = can be either = same properties = same molecule
what is a substituent?
atom of group of atoms which could be replaced by a hydrogen atom (hydrogen is not considered to be a substituent)
how do we find the amount of substituents for alkanes?
count substituents bonded to the carbons involved in the double bond (has to be around the double bond as we mean the alkene functional group, not the alkene molecule)
what is an E isomer?
An isomer that has the highest priority substituent atoms opposite side of the double bond
what is a Z isomer?
an isomer that has the highest priority substituent atoms on the same side of the double bond
how do we determine priority?
compare atomic number, largest = higher priority
if the atomic number for both substituents is equal, what does CIP rule tell us to do?
consider the atoms one bond further away (includes H) and compare those 2 atoms instead, so we compare the atoms with the 2nd highest atomic number, if those are equal, compare the atoms 3 bonds away etc and that whole substituent becomes the priority
how do we test for alkenes?
Add bromine
Bromine water is decolourised by the alkene (colourless)
what type of reaction is the test for alkenes?
addition reaction
what is an addition reaction?
a reaction in which one molecule combines with another to form a larger molecule with no other products
what are carbocations?
cations containing positively charged carbon
in addition reactions, what do alkenes form before the final product?
intermediates called carbocations
electrophilic addition reactions of alkenes with HBr?
1 pi bond breaks, electrons are attracted to delta positive hydrogen and a pair of e- forms a new bond with delta + hydrogen
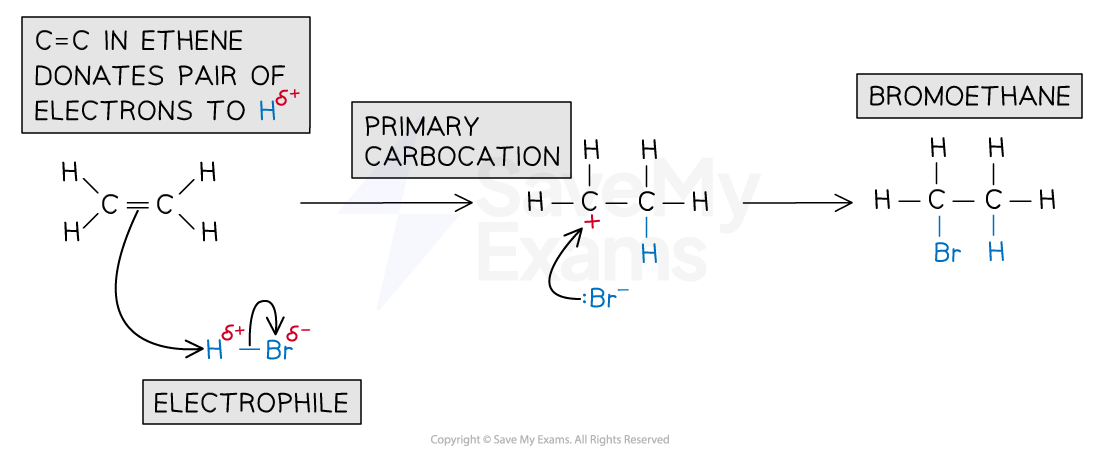
what is the electrophile for this reaction?
H-Br
electrophilic addition reaction of alkenes with Br2?
1 Br2 approaches, its electrons repel due to high electron (-ve) density of double bond = momentary partial charges occur, allowing Br to act as an electrophile
how is Br2 able to act as an electrophile?
as Br2 approaches, its electrons repel slightly due to the high electron density/ negative charge of the double C=C bond, so momentary partial charges occur, allowing Br to act as an electrophile
electrophilic addition of alkenes with H2SO4?
Alkene + concentrated sulfuric acid -> alkyl hydrogensulfate
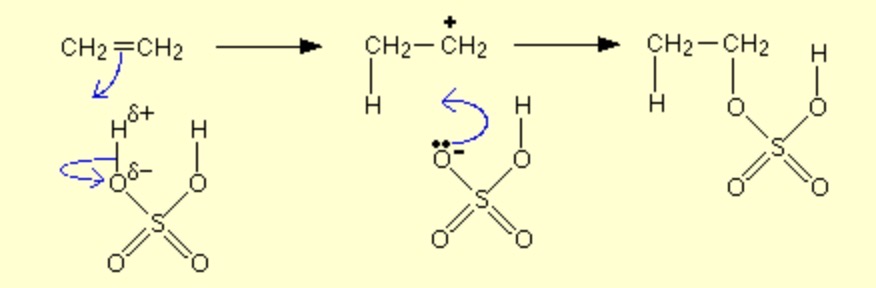
how are minor and major products formed in a reaction of unsymmetrical alkenes?
The major product is the one that is formed in greater quantity (as its more stable) and from a more stable carbocation intermediate.
The minor product is the one that is formed in lesser quantity and from a less stable carbocation intermediate
what is markovnikovs rule?
In an addition reaction of a hydrogen halide (HX) to an alkene, the halogen ends up bonded to the most substituted carbon atom.
what is an unsymmetrical alkene?
an alkene with different carbon chain lengths either side of the C=C double bond
true/false: major and minor products only form with unsymmetrical alkenes?
true x
when are carbocations most stable?
when the positively charged carbon is bonded to more alkyl groups (pictures in notes that help explain xx)
why does more alkyl groups help stabilise the carbocation?
alkyl groups donate electron density to the positive carbon, which stabilises it
what is a primary carbocation?
The positive carbon atom is attached to one alkyl group
what is a secondary carbocation?
The positive carbon atom is attached to two alkyl groups
what is a tertiary carbocation?
The positive carbon atom is attached to three alkyl groups
which carbocation is the most stable?
tertiary
what are addition polymers formed from?
Alkenes and substituted alkenes
define monomer
short chain molecule that joins with other monomers to form a polymer
define polymer
large, long chain macromolecule made up of thousands of small monomers joined together by a covalent bond
what is a repeating unit?
A part of a polymer whos repetition would produce the complete polymer if joined together, it is the shortest section of the polymer which is repeated
properties of addition polymers?
very long , hence strong intermolecular forces
strong and rigid ( due to strong intermolecular forces)
inert - chemically unreactive because the backbone of an addition polymer is a long chain of C-C single bond , which is strong and non polar
placed in landfills ( due to unreactivity)
solid at room temp. due to strong I.M. forces
all experience van der waals, some can have dipole-dipole or hydro
why are addition polymers solid at room temperatures?
very strong intermolecular forces
why are addition polymers often placed in landfills?
they are unreactive, so therefore not biodegradeable
steps to drawing the monomer of a polymer?
section off the repeating unit in the chain
add a double bond to the longest carbon chain
remove bonds between neighbouring pairs
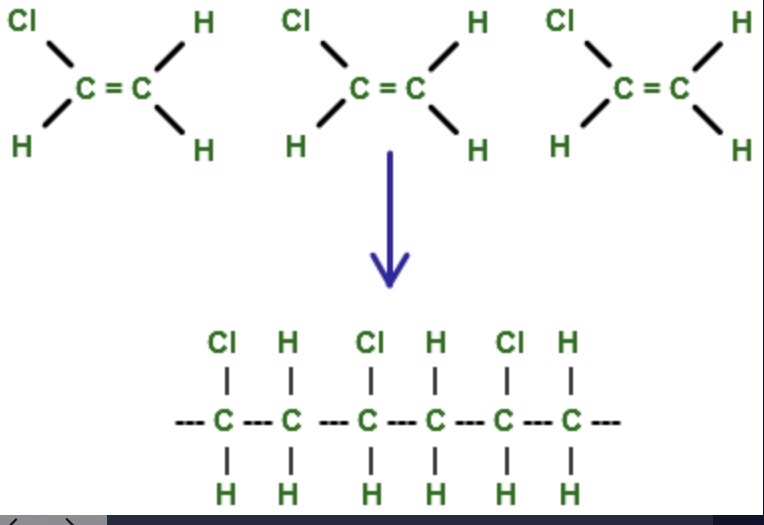
steps to drawing the repeating unit?
section off the repeating unit in the chain
place in a bracket with elongated side chains (to show its bonded to others/repeating), add an n to show its repeating
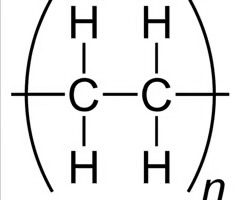
how to identify monomers/repeating unit in messy chains?
compare pairs of carbons on the main carbon chain
see which sets of pairs have the same substituents, if this isnt possible we know the polymers formed by 2 different monomers or more
what is a messy chain?
when monomers dont always combine together in neat patterns, they often bond together in inconsistent ways
uses of Poly(chloroethene) / PVC?
window frames, gutters, pipes, insulation for electrical wires, vinyls, plugs, bank cards, water bottles
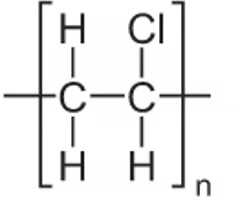
what is rigid PVC and properties?
a strong, stiff, low cost plastic material:
strong intermolecular forces make it stiff and inflexible
snaps if bent
uses of rigid PVC?
drainpipes, bank cards, plastic bottles, vinyls, plugs and window frames
how do chemists produce flexible PVC?
they add a plasticiser which acts as a spacer, separating the PVC chains, reducing the strength of the intermolecular forces between them, allowing it to bend without snapping (unlike rigid)
uses of flexible PVC?
wellington boots, cables, inflatable boats
explain the nature of intermolecular forces between molecules of polyalkenes
The Van der Waal's forces between the chains are very strong which results in the polymers having high melting and boiling points. The strength of van der Waals forces increase with chain length.
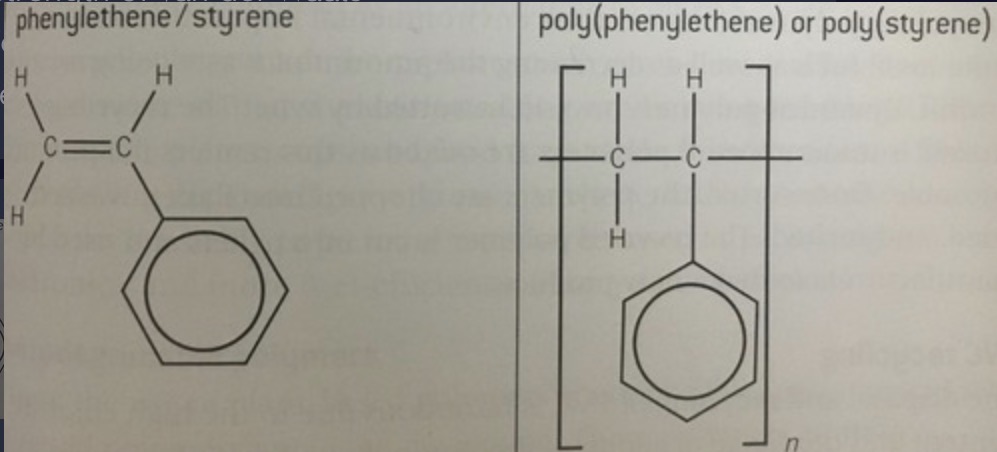
Making poly(phenylethene) (polystyrene) from phenylethene (styrene)