week 9 and 10
0.0(0)
0.0(0)
Card Sorting
1/99
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
100 Terms
1
New cards
carbohydrates
* make up the largest potion of the average North American diet
* they are the main energy source of carbohydrates
* they are the main energy source of carbohydrates
2
New cards
where is the main energy stored as in humans?
fats and lipids
3
New cards
monosaccharide
* single sugar
* sugars are in equilibrium between the straight chain molecule and cyclic molecules in solutions
* sugars are in equilibrium between the straight chain molecule and cyclic molecules in solutions
4
New cards
Di- and poly saccharides
* sugars molecules can be bonded together to form a larger sugar molecules
* the type of bond formed will determine the enzyme needed to break it down
* the type of bond formed will determine the enzyme needed to break it down
5
New cards
break down carbohydrates
* the first step is digestion
* various enzymes and processes in different areas of the digestive tract breakdown food to its simplest forms
* we can only absorb monosaccharides
* various enzymes and processes in different areas of the digestive tract breakdown food to its simplest forms
* we can only absorb monosaccharides
6
New cards
digestion of carbohydrates
* starts in the mouth with salivary amylase
* enzymatic breakdown of carbohydrates occurs by hydrolysis reactions
* enzymatic breakdown of carbohydrates occurs by hydrolysis reactions
7
New cards
when does carbohydrate digestion stop?
when it gets to the stomach. It is too acidic and salivary amylase becomes denatured
8
New cards
starch digestion
* begins to be broken down by salivary a-amylase, stops in the stomach and continues once the chyme leaves the stomach
* once the chyme leaves the stomach, bicarbonate is released by the pancreas along with pancreatic a-amylase to allow carbohydrate digestion to continue as the contents move into the duodenum
* once the chyme leaves the stomach, bicarbonate is released by the pancreas along with pancreatic a-amylase to allow carbohydrate digestion to continue as the contents move into the duodenum
9
New cards
what does bicarbonate do?
* helps to neutralize the acidic chyme from the stomach in the small intestine
10
New cards
what happens to starches as they start to get digested?
* they continue to break down into disaccharides
* enzymes in the brush border of the small intestine hydrolyze them to monosaccharides so they can be absorbed
* absorption into the capillaries takes the digested carbohydrates into the hepatic circulation and to the liver
* enzymes in the brush border of the small intestine hydrolyze them to monosaccharides so they can be absorbed
* absorption into the capillaries takes the digested carbohydrates into the hepatic circulation and to the liver
11
New cards
where are enzymes modified?
in the golgi complex
12
New cards
where are the carbohydrate-digesting enzymes?
* in the small intestine in integral membrane proteins
13
New cards
what keeps carbohydrate-digesting enzymes from being digested themselves? where in the cell does this happen?
* they are highly glycosylated (post-translational modification) to keep from being digested by the intestinal proteases
* the enzymes are distributed along the small intestine allowing the final monosaccharides to be absorbed
* any di/poly saccharides that are not digested may be metabolized by bacteria in the colon, producing gases, short chain fatty acids and lactate
* the enzymes are distributed along the small intestine allowing the final monosaccharides to be absorbed
* any di/poly saccharides that are not digested may be metabolized by bacteria in the colon, producing gases, short chain fatty acids and lactate
14
New cards
dietary fibre
* made up of undigestable carbohydrates
* enzymes are not able to break up the bonds (eg. cellulose
* enzymes are not able to break up the bonds (eg. cellulose
15
New cards
soluble types of fibre
* pectins and gums
* thought to reduce blood cholesterol levels binding bile salts or reducing reabsorption in the intestines
* slow down absorption of nutrients
* thought to reduce blood cholesterol levels binding bile salts or reducing reabsorption in the intestines
* slow down absorption of nutrients
16
New cards
what is an example of an insoluble fibre?
cellulose
17
New cards
lactase
* lactase is the enzyme that breaks down lactose to its monosaccharides, galactose and glucose
* lactase activity is highest in infants
* secondary lactase deficiency can result from injury to intestinal absorptive cells
* lactase activity is highest in infants
* secondary lactase deficiency can result from injury to intestinal absorptive cells
18
New cards
lactase non-persistence phenotype
* by adulthood, most of the worlds population have
19
New cards
lactase persistence phenotype
* continues consumption of daily products into adulthood has resulted in the continued expression of lactase at high levels
* this is rare
* this is rare
20
New cards
lactose intolerance
* lactose is a disaccharide
* in inability to breakdown and absorbed lactose, allows it to move to the large intestine where bacteria can metabolize it for energy
* it is metabolized to gases and lactic acid; this leads to symptoms associated with lactose intolerance
* eg. diarrhea also occurs due to the influx of water into the intestinal lumen
* can lead to dehydration and electrolyte imbalance if severe symptoms of diarrhea
* in inability to breakdown and absorbed lactose, allows it to move to the large intestine where bacteria can metabolize it for energy
* it is metabolized to gases and lactic acid; this leads to symptoms associated with lactose intolerance
* eg. diarrhea also occurs due to the influx of water into the intestinal lumen
* can lead to dehydration and electrolyte imbalance if severe symptoms of diarrhea
21
New cards
osmotic effect
* due to water moving into the large intestine to equalize the solute concentration between the cells and the intestine
22
New cards
why do glucose molecules need help to get into cells
* they are polar and so they require protein transporters to cross the lipid membrane
23
New cards
glucose transport
* glucose is a very polar molecule and therefore cannot freely diffuse through the lipid bilayer
* transporters are used to move glucose (and other monosaccharides) into the cells lining the small intestine
* the hydroxyl (OH) groups of glucose form hydrogen bonds with amino acids on the protein and are released into the interior of the cell
* transporters are used to move glucose (and other monosaccharides) into the cells lining the small intestine
* the hydroxyl (OH) groups of glucose form hydrogen bonds with amino acids on the protein and are released into the interior of the cell
24
New cards
SGLUT1
* moves glucose'/galactose and sodium from the small intestine to the mucosal cells
25
New cards
GLUT1
moves glucose from the blood into RBCs and across the blood-brain barrier
26
New cards
GLUT2
moves glucose from the mucosal cells to the blood, and from the blood into the liver and pancreas
27
New cards
GLUT3
moves glucose from the blood into neurons
28
New cards
GLUT4
moves glucose into muscle and adipose cells in response to insulin
29
New cards
GLUT5
* moves fructose from the small intestine to the mucosal cells
30
New cards
glucose in the brain
* glucose is transported into the brain at a rate just a little bit faster than it is used, this means there should always be enough glucose for the brain
* drops in blood glucose result in less glucose transport and therefore metabolism, resulting in the symptoms associated with hypoglycemia
* the brain uses mainly GLUT3, which is NOT insulin-sensitive
* drops in blood glucose result in less glucose transport and therefore metabolism, resulting in the symptoms associated with hypoglycemia
* the brain uses mainly GLUT3, which is NOT insulin-sensitive
31
New cards
lipoproteins
carry fatty acids that do not easily cross the BBB, that means glucose is the primary fuel for the brain and neurons
32
New cards
neural cells and glucose
1. tight junctions between endothelial cells
2. narrow intercellular space
3. lack of pinocytosis
4. continuous basement membrane
5. glucose transporters in both membranes
33
New cards
non-neuronal cells and glucose
1. no tight junctions
2. sometimes wide intercellular gaps
3. pinocytosis
4. discontinuous basement membrane
5. glucose can diffuse between cells and into interstitial fluid
34
New cards
treatment for diarrhea
* rehydration fluids for treatment need to contain glucose and sodium in order for absorption to occur
35
New cards
ATP
* commonly used as energy
* three phosphate bonds, when broken, release energy that energy can be used to do something esle
* three phosphate bonds, when broken, release energy that energy can be used to do something esle
36
New cards
how do we synthesize ATP?
* CELLS NEED TO HARNESS ENERGY FROM BREAKING OTHER CHEMICAL BONDS
37
New cards
ATP generation
* breaking the bonds in molecules allows cells to harness that energy to make ATP
* the energy is used for all other processes in the cell
* if the heart were not able to generate ATP, all its ATP would be depleted in less than 1 minute
* the energy is used for all other processes in the cell
* if the heart were not able to generate ATP, all its ATP would be depleted in less than 1 minute
38
New cards
glycolysis
* occurs in the cytoplasm
* present in all cell types
* phase 1: preparative phase
* starts with glucose
* 2 ATP are used to prepare this glucose molecule for breakdown into two pyruvates
* phase 2: ATP-generating phase
* break down generates 4 ATP directly
* 2 NADHs are generated which can be used to generate ATP later in the ETC using oxidative phosphorylation
* pyruvate can be broken down further via the TCA cycle generating even more NADH
* present in all cell types
* phase 1: preparative phase
* starts with glucose
* 2 ATP are used to prepare this glucose molecule for breakdown into two pyruvates
* phase 2: ATP-generating phase
* break down generates 4 ATP directly
* 2 NADHs are generated which can be used to generate ATP later in the ETC using oxidative phosphorylation
* pyruvate can be broken down further via the TCA cycle generating even more NADH
39
New cards
glycoloysis step 1-
* conversion of glucose to glucose-6-phosphate
* this traps the glucse in the cell
* once this happens, glucose-6-phosphate will be used by the cells to generate energy, stored (as glycogen), or used to make new molecules
* this traps the glucse in the cell
* once this happens, glucose-6-phosphate will be used by the cells to generate energy, stored (as glycogen), or used to make new molecules
40
New cards
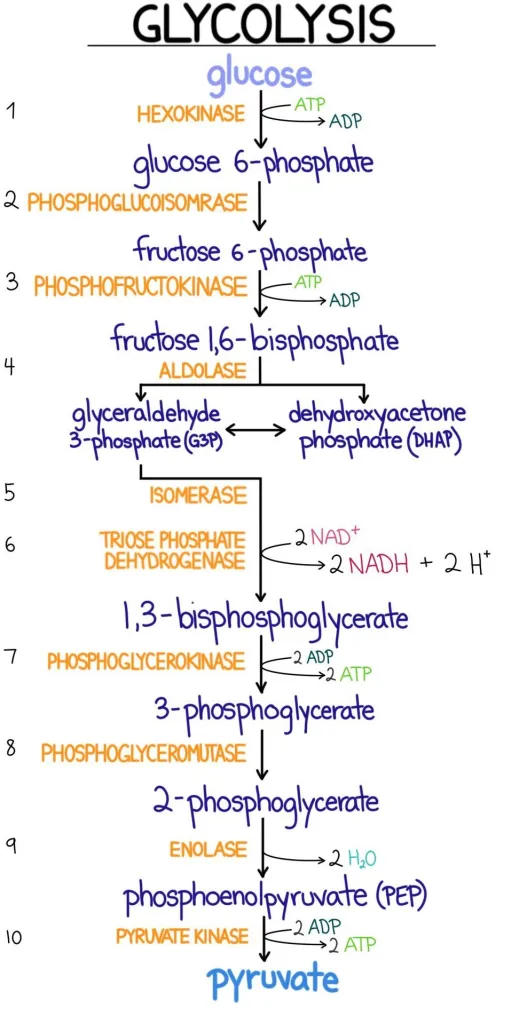
41
New cards
aerobic metabolism
* pyruvate is a branching point
* depending on the availability of oxygen, pyruvate will have a different fate
* with oxygen, pyruvate will enter the TCA cycle
* occurs in the mitochondria
* depending on the availability of oxygen, pyruvate will have a different fate
* with oxygen, pyruvate will enter the TCA cycle
* occurs in the mitochondria
42
New cards
anaerobic metabolism
* pyruvate will be converted into lactate
* this is needed to regenerate NAD+
* this generates lactate, which can build up in the tissues
* this is needed to regenerate NAD+
* this generates lactate, which can build up in the tissues
43
New cards
metabolic acidosis
* anerobic metabolism is often associated with lactic acidosis
* this is a misnomer as lactic acid is never generate
* may or may not occur when lactate levels are increased due to mitochondrial dysfunction
* important to note that H+ that causes this are not generated from the dissociated of lactic acid to lactate use of ATP results in the generating of ADP + Pi and H+
* this is a misnomer as lactic acid is never generate
* may or may not occur when lactate levels are increased due to mitochondrial dysfunction
* important to note that H+ that causes this are not generated from the dissociated of lactic acid to lactate use of ATP results in the generating of ADP + Pi and H+
44
New cards
uses of anaerobic metabolism
* certain tissues lack mitochondria since it might interefere with their function
* because of their function in oxygen transport, red blood cells obtain their energy from anaerobic glycolysis
* the tissues in the eye need to be free from light-deflecting structures and obtain most of their energy this way as well
* in skeletal muscle, when energy demand is high, anaerobic metabolism will kick in to help meet demands
* much less ATP is produced by anaerobic glycolysis (which occurs in the cytoplasm)
* tissues with low oxygen or few or no mitochondria (RBC) must use this method
* hypoxic tissues can use this pathway as well, over short term until oxygen delivery is restored, which is why lactate in the blood is used as an indicator of tissue perfusion
* because of their function in oxygen transport, red blood cells obtain their energy from anaerobic glycolysis
* the tissues in the eye need to be free from light-deflecting structures and obtain most of their energy this way as well
* in skeletal muscle, when energy demand is high, anaerobic metabolism will kick in to help meet demands
* much less ATP is produced by anaerobic glycolysis (which occurs in the cytoplasm)
* tissues with low oxygen or few or no mitochondria (RBC) must use this method
* hypoxic tissues can use this pathway as well, over short term until oxygen delivery is restored, which is why lactate in the blood is used as an indicator of tissue perfusion
45
New cards
glycolysis is fast but…
inefficient
46
New cards
mitochondria
* powerhouse of the cell
* site of the TCA cycle and the ETC
* there is an outer and inner membrane
* outer: oermeable to small ions
* inner: impermeable. This helps to establish a hydrogen ion gradient that can be used to drive ATP synthesis
* structure allows it to make a lot of ATP
* site of the TCA cycle and the ETC
* there is an outer and inner membrane
* outer: oermeable to small ions
* inner: impermeable. This helps to establish a hydrogen ion gradient that can be used to drive ATP synthesis
* structure allows it to make a lot of ATP
47
New cards
pyruvate dehydrogenase complex (PDC)
* moves the pyruvate from the cytoplasm to the mitochondria
* converts pyruvate to Acetyl CoA
* this generates NADH and a CO2 in the process
* converts pyruvate to Acetyl CoA
* this generates NADH and a CO2 in the process
48
New cards
tricarboxylic acid cycle (TCA)
* the oxidation of all the different fuels can generate acetyl coenzyme A, the 2-carbon substrate for the TCA
* also known as the krebs cycle
* occurs int the mitochondria
* the 2 carbons from acetyl CoA combine with 4 more in the cycle (oxaloacetate) to make the 6-carbon citric acid- because it is a cycle, the original 2 carbons are oxidized to CO2 and the 4-carbon oxaloacetate is regenerated
* all about generating NADH, FADH for the ETC
* also known as the krebs cycle
* occurs int the mitochondria
* the 2 carbons from acetyl CoA combine with 4 more in the cycle (oxaloacetate) to make the 6-carbon citric acid- because it is a cycle, the original 2 carbons are oxidized to CO2 and the 4-carbon oxaloacetate is regenerated
* all about generating NADH, FADH for the ETC
49
New cards
fuel oxidation
* all the different macronutrients can be metabolized to acetyl CoA
* acetyl CoA is the substrate for the TCA cycle
* acetyl CoA is the substrate for the TCA cycle
50
New cards
energy metabolism
phase 1: break down fuels/ Oxidization of fuels
phase 2: generate ATP from oxidative phosphorylation
* this is all about those NADH;s and FADH2
* THIS IS WHERE ALL THE OXYGEN COMES IN
* Most of the ATP we use in our body is generated in the ETC
phase 2: generate ATP from oxidative phosphorylation
* this is all about those NADH;s and FADH2
* THIS IS WHERE ALL THE OXYGEN COMES IN
* Most of the ATP we use in our body is generated in the ETC
51
New cards
oxidation and reducation
* reactions that drive energy generation
* loss of electrons= oxidation
* gain of electrons= reduction
* NAD and FAD are reduced in the TCA cycle
* NAD and FAD gain electrons from molecules in the pathway, which in turn become oxidized
* NADH and FADH2 are then oxidized in the ETC
* they donate electrons, converting back to NAD and FAD
* loss of electrons= oxidation
* gain of electrons= reduction
* NAD and FAD are reduced in the TCA cycle
* NAD and FAD gain electrons from molecules in the pathway, which in turn become oxidized
* NADH and FADH2 are then oxidized in the ETC
* they donate electrons, converting back to NAD and FAD
52
New cards
the electron transport chain
* aka. oxidative phosphorylation
* NADH donates to Complex I of the ETC
* this provides energy that can be used to move H+ out of the matrix and into the intermembrane space against its concentration gradient
* these electrons then get passed to CoQ, complex III, cytochrome c, and complex IV and finally to O2
* this process moves more and more H+ out of the matrix and into the intermembrane space
* this establishes an H+ gradient
* while some of this energy is used to move H+, a lot of the energy is last as heat- this is how we maintain our body temperature
* ATP synthase uses the movement of H+ back into the mitochondrial matrix WITH its concentration gradient to make ATP
* NADH donates to Complex I of the ETC
* this provides energy that can be used to move H+ out of the matrix and into the intermembrane space against its concentration gradient
* these electrons then get passed to CoQ, complex III, cytochrome c, and complex IV and finally to O2
* this process moves more and more H+ out of the matrix and into the intermembrane space
* this establishes an H+ gradient
* while some of this energy is used to move H+, a lot of the energy is last as heat- this is how we maintain our body temperature
* ATP synthase uses the movement of H+ back into the mitochondrial matrix WITH its concentration gradient to make ATP
53
New cards
how many ATP can be produced from 1 glucose molecule?
32-38 ATP
54
New cards
regulation of energy metabolism
* NADH has a major role in regulation of energy metabolism
* specifically the ratio of NADH/NAD
* a surplus of NADH indicated there is a lot of available energy in the cell
* an excess NAD indicates energy is being used up quickly
* if a cell still needs energy and its shut down, it becomes a deficit and goes into anerobic metabolism and eventually runs out of glucose
* specifically the ratio of NADH/NAD
* a surplus of NADH indicated there is a lot of available energy in the cell
* an excess NAD indicates energy is being used up quickly
* if a cell still needs energy and its shut down, it becomes a deficit and goes into anerobic metabolism and eventually runs out of glucose
55
New cards
mnemonic for krebs cycle
* can: citrate
* i: isocitrate
* ask: a-ketoglutarate
* some: succinyl CoA
* super: succinate
* fantastic: fumarate
* memes: malate
* on: oxaloacetate
* i: isocitrate
* ask: a-ketoglutarate
* some: succinyl CoA
* super: succinate
* fantastic: fumarate
* memes: malate
* on: oxaloacetate
56
New cards
what happens if we have hypoxia?
1. decreased mitochondrial ETC
2. decreased ATP and adenine nucleotides
3. increased Na
4. cellular swelling
5. increased plasma membrane permeability
6. increased Ca
7. mitochondrial permeability transition
8. Repeat 1
57
New cards
tissue hypoxia
low levels of oxygen in your body
58
New cards
tissue anoxia
* total lack of oxygen and chemicals such as cyanide can block utilization of oxygen, prevent energy generation and shut cells down (kill them)
59
New cards
what are the products of ATP hydrolysis?
ADP, inorganic phosphate and H+
60
New cards
OXPHOS diseases
* mitochondrial DNA is passed on from female gametes
* mutations in DNA that codes for mitochondrial proteins
* aggregate muscle tissue
* mutations in DNA that codes for mitochondrial proteins
* aggregate muscle tissue
61
New cards
steps of digestion
1. a small amount of lipid digestion occurs in the stomach due to lipases produced in the mouth and stomach
2. the liver produces bile, which is stored in the gallbladder and released into the small intestine to aid in the digestion and absorption of fat
3. the pancreas produces the enzyme pancreatic lipase, which is released into the small intestine to break down triglycerides into fatty acids and glycerol
4. in the small intestine, the products of fat digestion and bile form micelles, which move close enough to the brush border to allow lipids to diffuse into the mucosal cells
5. inside the mucosal cells, fatty acids are reassembled into triglycerides and incorporated into lipid transport particles, which enter the lymph
6. in the large intestine, unabsorbed fat is metabolized by bacteria. Very little fat is normally lost in the feces
62
New cards
triglycerides
* major dietary source of fat
* consist of a glycerol backbone with 3 fatty acids attached
* these are hydrolyzed to fatty acids and 2-monoacylglycerol in the small intestine
* lingual (mouth) and gastric (stomach) lipases are present but are most active with short and medium chain fatty acids of the type you would find in milk
* triglycerides are packaged into lipoproteins (chylomicrons) for transport in the body
* they are nonpolar and need the protein part to transport them
* consist of a glycerol backbone with 3 fatty acids attached
* these are hydrolyzed to fatty acids and 2-monoacylglycerol in the small intestine
* lingual (mouth) and gastric (stomach) lipases are present but are most active with short and medium chain fatty acids of the type you would find in milk
* triglycerides are packaged into lipoproteins (chylomicrons) for transport in the body
* they are nonpolar and need the protein part to transport them
63
New cards
bile salts
* not soluble in the aqueous environment of the intestine or the blood
* bile salts are synthesized from cholesterol in the liver
* act as emulsifiers to surround the fats and allow them to be broken down and absorbed
* secreted by the gallbladder where they are stores
* are amphipathic- hydrophobic on inside and hydrophilic on outside
* bile salts are synthesized from cholesterol in the liver
* act as emulsifiers to surround the fats and allow them to be broken down and absorbed
* secreted by the gallbladder where they are stores
* are amphipathic- hydrophobic on inside and hydrophilic on outside
64
New cards
bile salts → micelles
* bile salts are released form the gallbladder and enzymes are released from the pancreas due to the hormone cholecystokinin (CCK)
* CCK is released once the chyme enters the duodenum and in response to fats and proteins in the chyme
* bicarbonate is also released due to a signal from secretin to neutralize pH and allow the enzymes to function
* micelles form when the bile salts reach a concentration greater than the critical micelle concentration, below which the bile salts are soluble
* micelles form with polar heads around the outside surrounding the hydrophobic materials within
* CCK is released once the chyme enters the duodenum and in response to fats and proteins in the chyme
* bicarbonate is also released due to a signal from secretin to neutralize pH and allow the enzymes to function
* micelles form when the bile salts reach a concentration greater than the critical micelle concentration, below which the bile salts are soluble
* micelles form with polar heads around the outside surrounding the hydrophobic materials within
65
New cards
lipid digestion
* the enzyme colipase is released by the pancreas and binds to the lipids and bile salts around emulsion droplets
* the lipase can then break down the triglycerides (TGs) in the micelles into fatty acids and monoglycerides
* the lipase can then break down the triglycerides (TGs) in the micelles into fatty acids and monoglycerides
66
New cards
recycling of bile salts
* the contents are absorbed from the intestines into the mucosal cells, but the bile salts are left behind
* the bile salts are reabsorbed further down the intestine to be used again in another digestive cycle
* the bile salts are reabsorbed further down the intestine to be used again in another digestive cycle
67
New cards
medium and short chain fatty acids
* do not require bile salts for absorption
* they are smaller and more water soluble
* The polar acid group makes up a larger proportion of the molecule, allowing water to form a hydration shell around it
* they can enter the blood and are transported to the liver bound to albumin
* long chain fatty acids are not soluble in water and must be transported in other ways
* they are smaller and more water soluble
* The polar acid group makes up a larger proportion of the molecule, allowing water to form a hydration shell around it
* they can enter the blood and are transported to the liver bound to albumin
* long chain fatty acids are not soluble in water and must be transported in other ways
68
New cards
lipid transport: chylomicrons
* fat containing molecules within the blood that have come from the digestive tract
* once the fats have been digested and absorbed into the mucosal cells, they are reassembled into triglycerides and packaged with protein to allow travel in water based blood
* the chylomicrons first travel into the lymphatic system and enter the bloodstream through the thoracic duct
* in the mucosal cells lining the small intestine, the digested fat moves into the smooth ER and are reassembled into triglycerides
* it then combines with the APoB-48, the protein component of the lipoprotein
* the nascent chylomicrons are transported to the cell surface in the vesivles
* these secreted particles enter the lymph system through the lacteals
* once the fats have been digested and absorbed into the mucosal cells, they are reassembled into triglycerides and packaged with protein to allow travel in water based blood
* the chylomicrons first travel into the lymphatic system and enter the bloodstream through the thoracic duct
* in the mucosal cells lining the small intestine, the digested fat moves into the smooth ER and are reassembled into triglycerides
* it then combines with the APoB-48, the protein component of the lipoprotein
* the nascent chylomicrons are transported to the cell surface in the vesivles
* these secreted particles enter the lymph system through the lacteals
69
New cards
lipoprotein lipase (LPL)
* an enzyme that removes triglycerides from chylomicrons
* lines the capillaries in muscle and adipose tissues, breaking down triglycerides into fatty acids that can be absorbed by surrounding cells
* the chylomicron remnants travel to the liver for disposal/recycling by lysosomes
* lines the capillaries in muscle and adipose tissues, breaking down triglycerides into fatty acids that can be absorbed by surrounding cells
* the chylomicron remnants travel to the liver for disposal/recycling by lysosomes
70
New cards
fate of fatty acids
* fatty acids are stored in the body as triglycerides, an efficient and lightweight method of energy storage
* because fatty acids are more reduced than carbohydrates, they provide more energy (glucose is already partially oxidized)
* fatty acids are the main source of energy during fasting, but not all tissues can use this fuel
* because fatty acids are more reduced than carbohydrates, they provide more energy (glucose is already partially oxidized)
* fatty acids are the main source of energy during fasting, but not all tissues can use this fuel
71
New cards
fatty acid breakdown
* B-oxidation of fatty acids occurs with long chain fatty acids, the most common component of our diets
* it is called B-oxidation because the bond between the a- and B-carbon is broken in successive rounds to create 2-carbon acetyl CoA molecules that can enter the TCA cycle
* it is called B-oxidation because the bond between the a- and B-carbon is broken in successive rounds to create 2-carbon acetyl CoA molecules that can enter the TCA cycle
72
New cards
fatty acid synthesis
* in the liver, excess carbohydrates are converted into fatty acids for storage
* the starting material for this is Acetyl-CoA
* fatty acid synthesis takes excess Acetyl-CoA and combines them into a larger molecule
* this excess fatty acid can be sent to other tissues to be used for energy or to adipose tissue for storage
* product: ACP
* the starting material for this is Acetyl-CoA
* fatty acid synthesis takes excess Acetyl-CoA and combines them into a larger molecule
* this excess fatty acid can be sent to other tissues to be used for energy or to adipose tissue for storage
* product: ACP
73
New cards
what are the different types of lipoproteins?
* very low density lipoprotein (VLDL)
* intermediate density lipoprotein (IDL)
* low density lipoprotein (LDL)
* high density lipoprotein
* intermediate density lipoprotein (IDL)
* low density lipoprotein (LDL)
* high density lipoprotein
74
New cards
lipoproteins in digestion
1. chylomicrons formed in the mucosal cells pass into the lymph, which drains into the blood
2. lipoprotein lipase breaks down the triglycerides in chylomicrons into fatty acids and glycerol, which can then enter the surrounding cells
3. chylomicron remnants travel to the liver, where they are disassembled
4. VLDLs transport lipids away from the liver. At the tissues, lipoprotein lipase breaks down the triglycerides in VLDLs and the fatty acids are absorbed into the cell
5. IDL particles are either returned to the liver or transformed in the blood into LDL particles
6. LDL particles bind to LDL receptors, which transport them into the cell, where the cholesterol and other components can be used
75
New cards
VLDL → IDL → LDL
* VLDL is produced by the liver to circulate excess triglycerides to the body and adipose tissue
* VLDL is produced mainly from excess carbohydrates in the liver (fatty acid synthesis)
* VLDL has mostly triglycerides and relatively low amounts of protein, cholesterol and phospholipids
* lipoprotein lipase will remove triglycerides from VLDL
* as the triglycerides are removed, VLDL becomes IDL
* IDL can go back to the liver or continue to have triglycerides removed, eventually becoming LDL- the bad cholesterol
* VLDL is produced mainly from excess carbohydrates in the liver (fatty acid synthesis)
* VLDL has mostly triglycerides and relatively low amounts of protein, cholesterol and phospholipids
* lipoprotein lipase will remove triglycerides from VLDL
* as the triglycerides are removed, VLDL becomes IDL
* IDL can go back to the liver or continue to have triglycerides removed, eventually becoming LDL- the bad cholesterol
76
New cards
what happens with glucose in the fed state?
* in the fed state, glucose maintains optimal blood levels and glycogen stores are replenished
* any remaining glucose is converted to triglycerides and packaged into VLDLs with ApoB-100 for release into the bloodstream
* any remaining glucose is converted to triglycerides and packaged into VLDLs with ApoB-100 for release into the bloodstream
77
New cards
cholesterol
* insoluble in water and must be transported thought the blood within lipoprotein
* important molecule in our cells
* stabilizes cell membrane
* precursor of the sex hormones and vitamin D
* used to make bile salts
* one of the most well-recognized molecules because of its relationship with heart disease
* important molecule in our cells
* stabilizes cell membrane
* precursor of the sex hormones and vitamin D
* used to make bile salts
* one of the most well-recognized molecules because of its relationship with heart disease
78
New cards
LDL and cholesterol
LDL lipoproteins have a relatively high amount of cholesterol and high levels of LDL may indicate excess cholesterol is available
79
New cards
LDL vs HDL
* cells with LDL receptors can bind to LDL particles adn engulf them, allowing the, to use the cholesterol inside
* these cells can then release “empty” HDL particles
* HDL particles participate in reverse cholesterol transport
* they can soak up cholesterol from vascular cells and returning it to the liver
* HDL are the good cholesterol
* these cells can then release “empty” HDL particles
* HDL particles participate in reverse cholesterol transport
* they can soak up cholesterol from vascular cells and returning it to the liver
* HDL are the good cholesterol
80
New cards
atherosclerosis
* LDL lipoproteins are transported to tissues to deliver cholesterol to cells by a receptor mediated process
* when there are lots of LDL lipoproteins in the blood, all the receptors become saturated and LDL accumulates in the blood
* exposure of the vascular endothelial cells to high LDL levels may start the inflammatory process that leads to atherosclerosis
* the problem is too much LDL
* when there are lots of LDL lipoproteins in the blood, all the receptors become saturated and LDL accumulates in the blood
* exposure of the vascular endothelial cells to high LDL levels may start the inflammatory process that leads to atherosclerosis
* the problem is too much LDL
81
New cards
protein digestion
* begins in the stomach (other than mechanical breakdown by the teeth in the mouth)
* HCL (released by the gastric parietal cells) first denatures the proteins to make them easier for pepsin to cleave
* HCL (released by the gastric parietal cells) first denatures the proteins to make them easier for pepsin to cleave
82
New cards
why would HCL in stomach acid cause a protein to become denatured?
all our proteins are inactive
83
New cards
pepsinogen
* secreted by chief cells in the stomach
* zymogen form of pepsin
* HCL in the stomach causes pepsinogen to change confirmation and cleave itself (autocatalysis), becoming the active form pepsin
\
* zymogen form of pepsin
* HCL in the stomach causes pepsinogen to change confirmation and cleave itself (autocatalysis), becoming the active form pepsin
\
84
New cards
digestive enzymes
* once food leaves the stomach, the pancreas releases enzymes as well as bicarbonate ions to neutralize the acidic chyme allowing enzymes in the intestine to function
* the pancreatic proteases are all released as zymogens so that they do not digest each other or other proteins in the cell or pancreas
* the pancreatic proteases are all released as zymogens so that they do not digest each other or other proteins in the cell or pancreas
85
New cards
why is it important that enzymes are inactive until they reach the intestine?
it can cause pancreatitis
86
New cards
trypsinogen
* cleaved to trypsin by enteropeptidase secreted by the brush border cells
87
New cards
trypsin
* cleaves the other zymogens to activate them in the intestine, where they break proteins down to di- and tri-peptides
88
New cards
endopeptidases
* cleave the peptide bonds BETWEEN two amino acids
* each peptidase cleaves the peptide bond around a particular type of amino acid
* each peptidase cleaves the peptide bond around a particular type of amino acid
89
New cards
exopepitdase
* cleave the peptide bond at the end of a polypeptide, releasing a single amino acid
* found at the brush border and within the intestinal cells
* they finish the job proteolytic cleavage into individual amino acids
* found at the brush border and within the intestinal cells
* they finish the job proteolytic cleavage into individual amino acids
90
New cards
amino acid absorption
* amino acids transport is similar to glucose transport in the digestive cells
* absorption is by secondary active transport, along with sodium ions
* there are at least 6 different amino acid transporters… some overlap
* they each have specifity for similar amino acids- each amino acid is usually transported by more than one carrier
* intestinal cells that are shed are also digested as are the digestive enzymes themselves
* all this protein is eventually broken down to amino acids and absorbed in the intestine
* once in the blood, amino acids travel to the liver and are distributed from there for protein synthesis
* absorption is by secondary active transport, along with sodium ions
* there are at least 6 different amino acid transporters… some overlap
* they each have specifity for similar amino acids- each amino acid is usually transported by more than one carrier
* intestinal cells that are shed are also digested as are the digestive enzymes themselves
* all this protein is eventually broken down to amino acids and absorbed in the intestine
* once in the blood, amino acids travel to the liver and are distributed from there for protein synthesis
91
New cards
amino acid pool
\*\*\* proteins cannot be stored in our body
* we have 3 uses
1. energy production
2. synthesis of glucose or fatty acids
3. synthesis of nonprotein molecules that contain nitrogen
* we have 3 uses
1. energy production
2. synthesis of glucose or fatty acids
3. synthesis of nonprotein molecules that contain nitrogen
92
New cards
can amino acids be created?
* no they are obtained form our died
93
New cards
essential amino acids
* our cells cannot make them, and they must be taken in from our diet
94
New cards
conditionally essential amino acids
* if we have the starting materials, we can make them
95
New cards
amino acid metabolism
* more complicated because amino acids contain nitrogen
* in addition to being used to make new proteins and broken down for energy, amino acids can be used to make nitrogen-containing compounds
* nitrogen can also be toxic, so our bodies need to get rid of the excess
* in addition to being used to make new proteins and broken down for energy, amino acids can be used to make nitrogen-containing compounds
* nitrogen can also be toxic, so our bodies need to get rid of the excess
96
New cards
nitrogen balance
nitrogen intake=nitrogen output
* total body protein does not change
* total body protein does not change
97
New cards
negative nitrogen balance
nitrogen intake < nitrogen output
* total body protein decreases
* total body protein decreases
98
New cards
positive nitrogen balance
nitrogen intake > nitrogen output
* total body protein increases
* total body protein increases
99
New cards
albumin
* most abundant blood protein, it is made in the liver, as are many other blood proteins
* makes up about 60% of total plasma protein and is thought to contribute 70-80% of total osmotic pressure of plasma
* in conditions of protein malnutrition, albumin synthesis is decreased quickly
* binds fatty acids and many drugs
\
* makes up about 60% of total plasma protein and is thought to contribute 70-80% of total osmotic pressure of plasma
* in conditions of protein malnutrition, albumin synthesis is decreased quickly
* binds fatty acids and many drugs
\
100
New cards
what are some solutions to low levels of albumin?
* IV treatment