Product Testing: Stability
1/50
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
51 Terms
Why do we need to do stability testing?
Pharmaceutical products deteriorate upon storage
All products have a shelf life / expiry date, which you need to know as a pharmacist
How do we formulate medicines?
To minimise degradation
Possible routes of degradation
Oxidation
Photodegradation
Heat
Solvent degradation
Acid and base catalysis
Temperature and degradation
Increase in temperature = increase in rate of degradation
Can refrigerate (2-8oC) a product if unstable at room temp
Or freeze (<-15oC)
Temperature and degradation
What is the problem with freezing medicines?
If heat is used for thawing = degradation
As the product melts = we end up with interfaces = proteins stick to interfaces, unfold and lose activity
Some drugs e.g. amoxicillin less stable when frozen than in the fridge » as changes solubility
Biopharmaceuticals and vaccines can be degraded by freezing
Temperature and degradation
Where is the most risk of exposure to elevated temps
Transport and storage in vehicles
What is chemical degradation?
Any process where covalent bonds are broken
So chemical structure changes
What can undergo chemical degradation?
Both the active drug AND excipients
There may be risk of the API reacting with the excipients
What group of chemicals are most drugs?
What is the problem with this?
Most drugs are esters or amides
If water is present, these can hydrolyse to alcohols and carboxylic acids
How can we prevent hydrolysis of drugs that contain esters and amides?
Changing the solvent / the dosage form
Can replace water with another solvent
We can choose this solvent based on the dielectric constant (a measure of polarity) » influences how fast charged species react
Need to consider toxicity of solvent and compatibility with the drug (ensure it doesn’t react with the drug)
Using a solid dosage form instead
Solid dosage forms more stable than liquid dosage form » as in a solution, every single molecule of drug is surrounded by water
But: reactions can occur in water absorbed onto the surface of the particle
E.g. poorly stored aspirin tablets smell of acetic acid (vinegar)
Freeze-dried powders and then reconstituted just before use
For drugs that are very unstable e.g. penicillin
Formulate as a suspension
Suspensions more stable than solutions
Drug molecules in the centre of the particle protected from water
Drug molecules on the surface may react with water
Why should you not store medicines in the bathroom?
Steam = water adsorbs to the surface of particles = hydrolysis
How can we prevent hydrolysis of drugs that contain esters and amides?
Changing the solution itself
Why is pH of the solution important?
A catalyst speeds up a reaction without being consumed itself » so a small amount of catalyst can cause a lot of degradation
H3O+ and OH- (from the dissociation of water) can catalyse hydrolysis processes
» so the drug will degrade faster at acidic or basic pH values
How can we prevent hydrolysis of drugs that contain esters and amides?
Changing the solution itself
Graph of pH and degradation rate
Specific acid hydrolysis: hydrolysis catalysed by presence of H+
Specific base hydrolysis: hydrolysis catalyse by presence of OH-
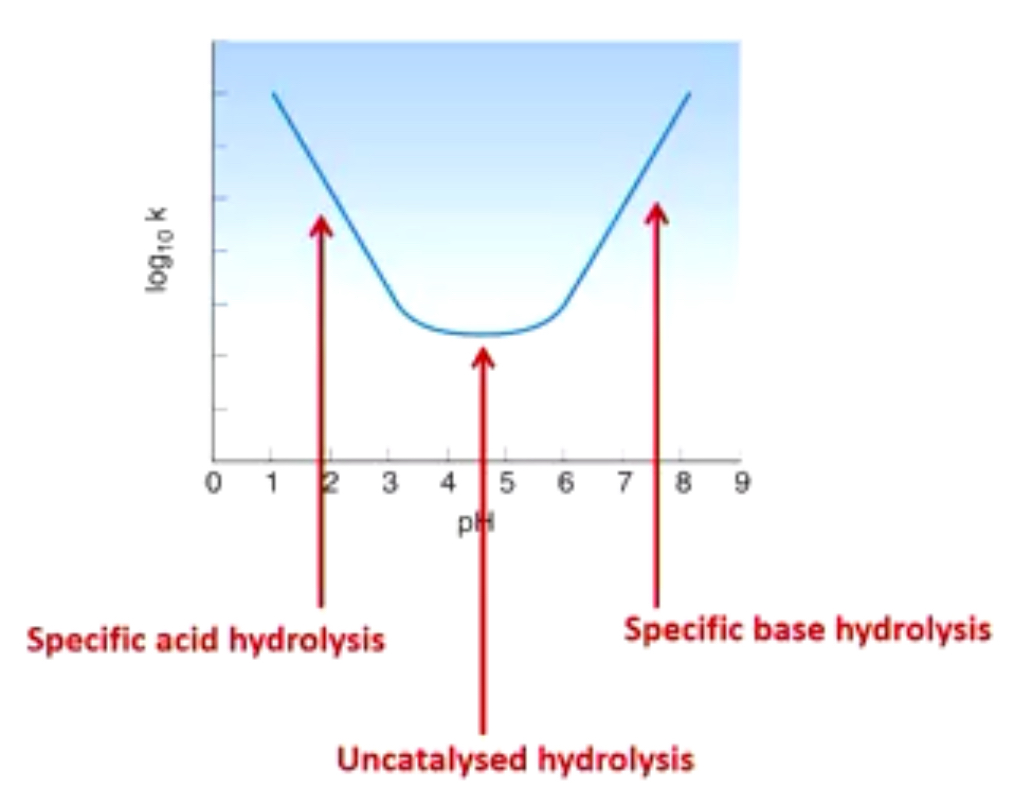
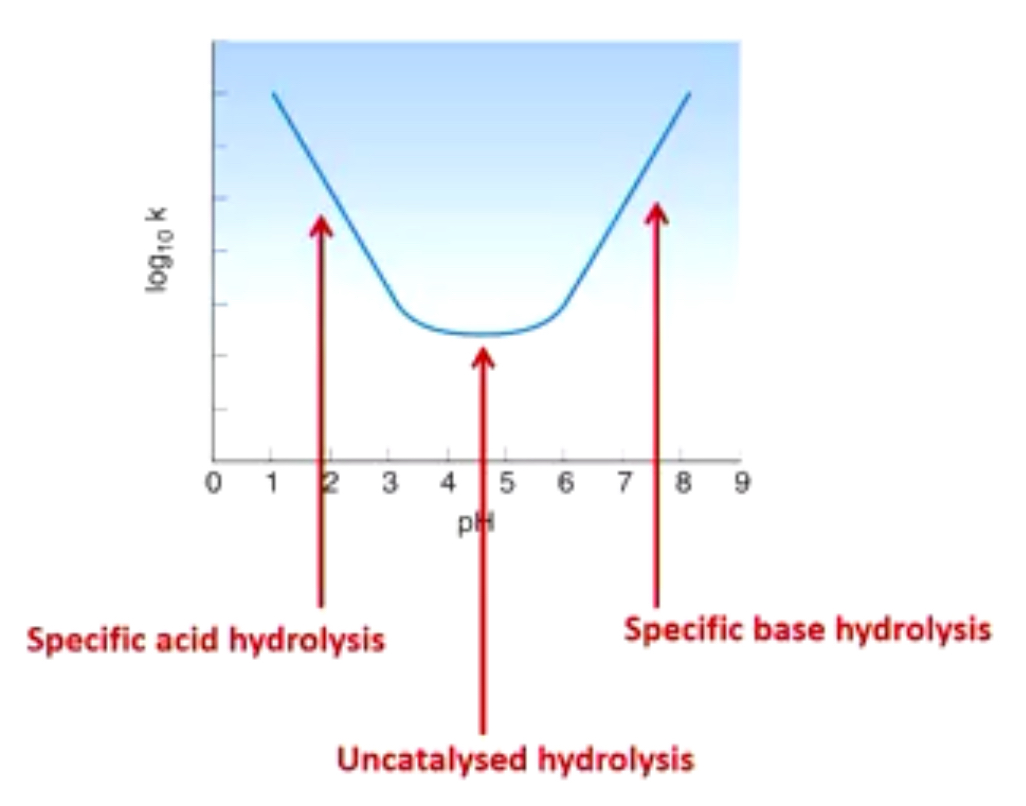
How can we prevent hydrolysis of drugs that contain esters and amides?
Changing the solution itself
Why is this graph important?
Tells us the pH range where degradation rate is slowest (uncatalysed hydrolysis)
Tells us when we need to add a buffer
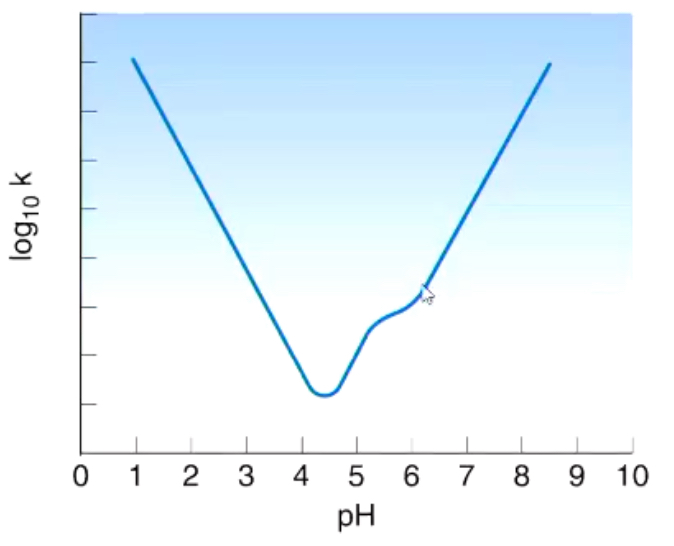
How can we prevent hydrolysis of drugs that contain esters and amides?
Changing the solution itself
What is this curve showing?
Many drugs ionise
And the neutral and charged forms of the drug degrade at different rates » hence, there is a point of inflection
How can we prevent hydrolysis of drugs that contain esters and amides?
Changing the solution itself
Specific vs General catalysis
Specific catalysis: H3O+ and OH- catalysing degradation
General catalysis: other species catalysing degradation
How can we prevent hydrolysis of drugs that contain esters and amides?
Changing the solution itself
Examples of general catalysis
Ions in buffers
E.g. phosphate or acetate buffer catalyses the hydrolysis of chloramphenicol = incompatible with the drug
Can use borate buffer instead which does not catalyse degradation = compatible with the drug
Light and degradation
Light causes degradation
To protect from light:
Keep medicine in a tinted glass container / opaque outer container e.g. cardboard
To block out UV light (which causes degradation)
Oxygen and degradation
Oxygen causes degradation
To protect from oxygen (oxidation):
We can flush the containers with an inert gas e.g. N2, Ar, CO2 » to expel all the air and oxygen from the container
However:
hard to remove all oxygen
best used for single-use containers as with multi-use, opening and closing allows for gas exchange and oxygen to enter
Oxygen and degradation
When does oxidation occur the fastest?
So how can we further prevent oxidation?
Oxidation is accelerated at high pH
So we can formulate at low pH
» However, there is a trade off between minimising oxidation and minimising hydrolysis
Metal ions e.g. Cu2+ and Fe3+ catalyse oxidation
These are present in trace amounts in all formulations
However they are not used up so can cause a lot of degradation
We can add a chelating agent, like EDTA, to bind to the metal ion and prevent catalytic activity
Add an an antioxidant
Reacts with O2 and removes it from the formulation
Stops free radical reactions
E.g. ascorbic acid (water soluble)
E.g. a-tocopherol (oil soluble)
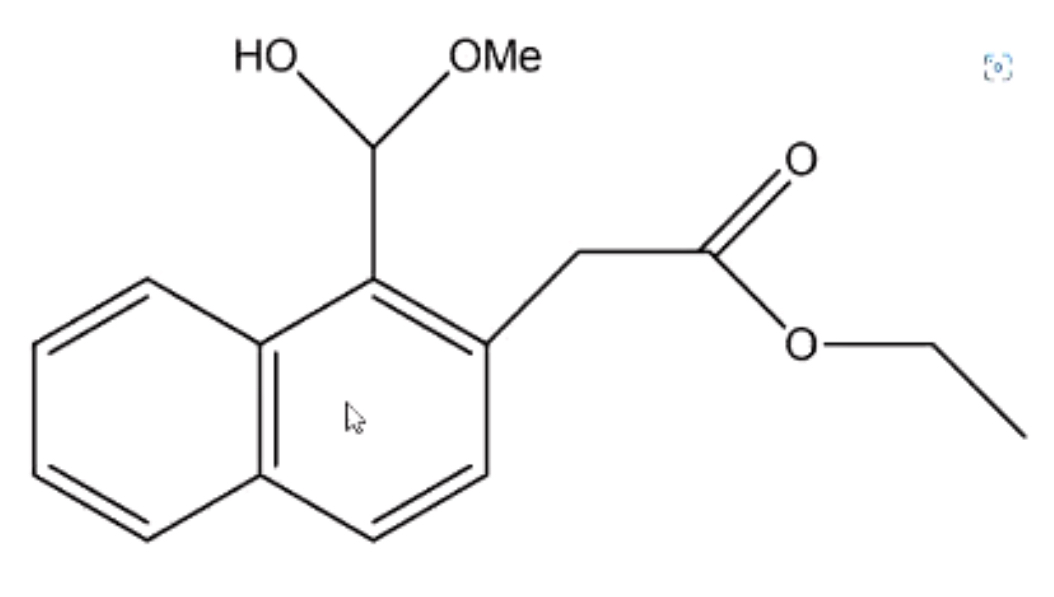
EQ:
What degradation might this drug undergo?
Hydrolysis
Oxidation (of OH group to a ketone)
Heat degradation
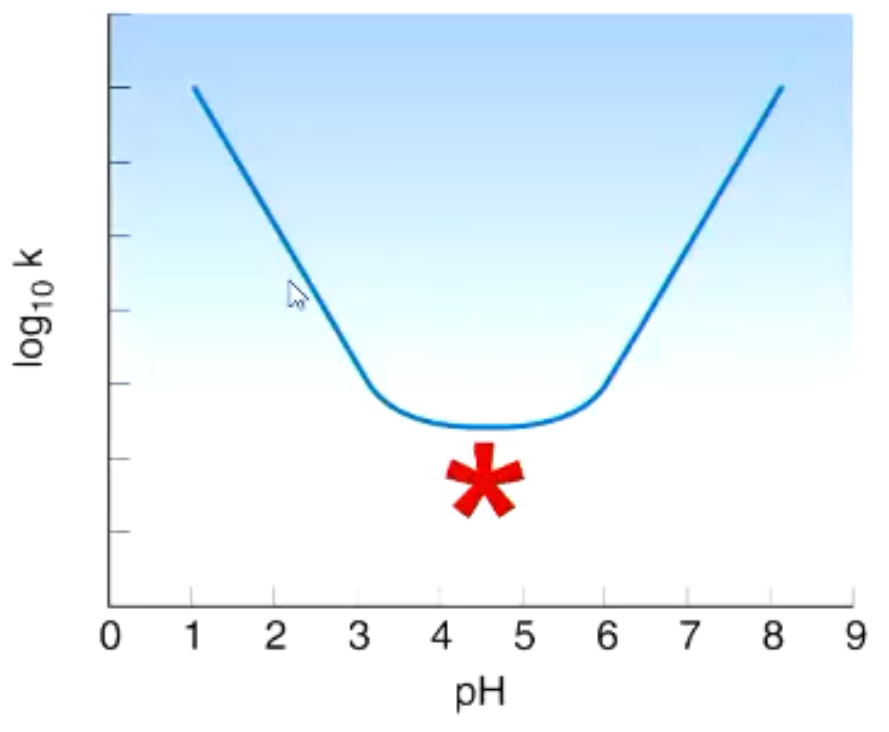
EQ:
What does the region marked * on the diagram correspond to?
Uncatalysed hydrolysis
Physical Stability
Problems of containers for liquid dosage forms?
Sorption of drug to container » leads to loss of drug
Shedding of particles from glass container » poor appearance, poor mouth-feel (affects adherence)
Extraction of materials from container into liquid » toxicity, change of pH
Evaporation of volatile components e.g. peppermint » poor taste
Physical Stability
2 types of sorption that can occur
Adsorption: particles of drug adhere to the walls of the container
Absorption: molecules of drug taken up inside the wall of the container
» both cause the loss of drug from the solution
Physical Stability
When is sorption more likely to occur?
Non-polar molecules have a high affinity for plastics and rubbers
e.g. Diazepam in solution sorbs to plastic packaging
pH can affect sorption » unionised form of the drug is less polar so more sorption to plastics and rubbers
Physical Stability
How can we prevent problems to do with containers for liquid dosage forms?
Shedding of glass particles » use a plastic container
If drug is hydrophobic and will stick to walls of a plastic container (non-polar) » use a glass container (polar)
Evaporation of volatile compounds » don’t use rubber caps/lids
Physical Stability
Physical instability of solutions
Precipitation of the drug = loss of efficacy, inaccurate dosing
Precipitation of its degradation products = poor appearance
Physical Stability
Physical instability of suspensions
Caking
Ostwald ripening » even if drug is poorly water soluble, little bits of the drug may dissolve and may precipitate out
» can lead to inaccurate dosing, poor appearance, grittiness
Physical Stability
Physical instability of emulsions
Creaming » dispersed phase droplets accumulate at the top
Cracking » emulsion breaks into 2 separate phases
» poor appearance (affects adherence)
Reduction in viscosity
» difficult application
» non-homogenous product
» increased risk of creaming and cracking
Physical Stability
Effect of changes in physical property
Compromises appearance » can reduce adherence
Compromises efficacy » inaccurate dosing, less drug present
What do we mean by preformulation?
Understanding the properties of the drug
We do this using qualitative measurements of the drug’s susceptibility to oxidation, hydrolysis and light degradation
Stability Testing: Preformulation
How do we measure the drug’s susceptibility to oxidation?
Heat solutions of the drug with / without oxygen flushing
Then look at chemical structure of the drug for degradation
Stability Testing: Preformulation
How do we measure the drug’s susceptibility to hydrolysis?
Heat solutions of the drug in water, acid and base
Stability Testing: Preformulation
How do we measure the drug’s susceptibility to light degradation?
Shine artificial daylight lamps on the drug
Stability Testing: Preformulation
What other preformulation test do we do?
Investigate stability of the drug in the solid state
Keep the drug at high temp
Add the drug with possible excipients
Stability Testing: Stress Testing
High temperatures
What does this involve?
Storing the drug at high temperatures
We then calculate the rate of degradation at RT using the Arrhenius equation:
k = Ae-Ea/RT
You then plot a graph of k vs the different temperatures
Calculate equation of the line
Determine what the rate of reaction at room temperature should be
Carry out with the drug alone then repeat with drug + excipients then with candidate formulations (exactly how the patient will take the drug)
Stability Testing: Stress Testing
High temperatures
Advantages
Speeds up the degradation process instead of waiting for years For degradation to happen at room temp
So we get results quickly
Stability Testing: Stress Testing
High temperatures
Limitations
Predicted shelf life at room temp is inaccurate
Different reactions may occur at higher temperatures than room temperature
Degradation products may themselves break down
E.g. if trying to quantify the amount of degradation product (B) but the degradation product (B) breaks down (C), we will not be able to quantify the C as we are only looking for the B
Heating reduces moisture levels in solid products
Underestimate rates of hydrolysis
The stability of the product must be studied in the actual storage conditions to be used
Stability Testing: Stress Testing
Temperature Cycling
What does this involve?
Subjecting liquid products to freeze/thaw cycles (very high then very low temps)
This can cause:
Ageing and particle growth in suspensions,
Cracking in emulsions
Precipitation in solutions
Stability Testing: Stress Testing
Photostability Testing
What does this involve?
Shine an artificial daylight lamp on pure drug
Then repeat with formulated product
Then use same packaging as will be used when drug is released to market
Stability Testing: Long Term Testing
What is long-term testing?
Long-term studies performed under actual storage conditions
After preformulation and stress testing, we should now have a formulation that we are confident is stable
Long-term testing is used to validate this to show a regulator that the drug is ready to be released to market
Stability Testing: Long Term Testing
What do we do during long-term testing?
Use worst case scenarios of temperature and humidity E.g. in particular regions of the world, how hot and how humid does it get
Use packaging that is intended to be used in the market
Samples are removed for 12 months
We then assay for both the drugs and excipients
Might also need pH tests, microbial tests and physical characteristic tests
Stability Testing: Long Term Testing
Requirements for long-term testing?
Use same packaging for testing as will be used for the product
Test at least 3 batches of the product
Store liquid formulations inverted to ensure that the contents interact with the cap of container
Sample every 3 months in year 1, every 6 months in year 2 and then annually until the end of the proposed shelf life
Stability Testing: Long Term Testing
How big are the samples used?
Batches of at least pilot scale
Stability Testing: Long Term Testing
What are we looking for when we sample every 3 months/6 months/annually?
Oral solutions, suspensions, emulsions:
Precipitation
Clarity (solutions)
pH
Viscosity
Extractables
Microbial contamination
Suspensions:
Dispersibility
Rheological properties
Size and distribution of particles
Emulsion:
Phase separation
Size distribution of dispersed phase
Stability Testing: Long Term Testing
Criteria for results
Degradation must leave >90% of stated dose left » use statistical tests
Appearance, smell and taste must be acceptable
Test at least 3 batches, calculate shelf lives and take the shortest shelf life
When do we NOT need to control humidity in stress testing / long term testing?
For aqueous products in glass containers
Purpose of pre-formulation and stress testing summary
Preformulation: tells us which degradation will pose the biggest problem
Stress testing: tells us the rate of degradation and quick information about the stability of the drug
EQ:
Explain the differences between the four global climactic zones. Why is it important to study product stability in all the zones where the product will be sold?
The four zones differ in their average temperatures and relative humidities.
It is important to study product stability in all the zones where a product will be sold in order to ensure that it is stable in that set of conditions: temperature and relative humidity (RH) will affect degradation rates and processes.
EQ:
What is caking, and why is it problematic in a pharmaceutical suspension?
Caking arises when particles in a suspension sink very slowly
This results in minimal liquid trapped within the particle
So the particles are very difficult to disperse upon shaking
This means most of the drug will be stuck at the bottom of the container
Leading to inaccurate dosing
EQ:
Why do we test multiple batches of a product during long-term stability testing?
There will be batch to batch variability - not all batches will be identical
So we need to test several batches to ensure we have a good overall understanding of how the formulation will behave