CHEM12 - unit 4A - acids + base part 1
1/49
Earn XP
Description and Tags
part one - acids and base part 1
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
50 Terms
arrhenius theory
acid
base
salt
acid: formula starts with H → releases H+ in solution
base: formula ends with OH → releases OH- in solution
salt: ionic compound produced from a neutralization reaction
HA + BOH → BA + H2O
acid properties (irl)
turns litmus paper red
corrosive
sour
react w/ metals
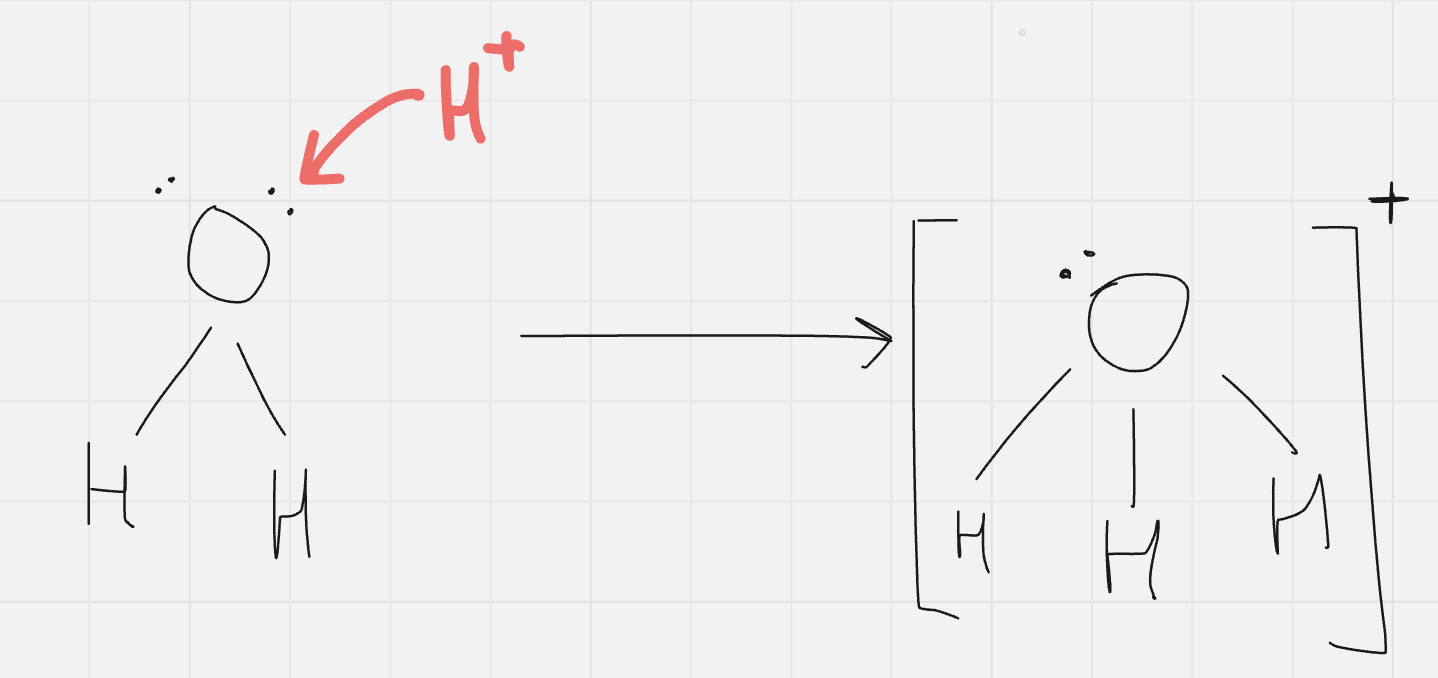
what do H+ form in water
since positive charge of H+ strongly attracted to negatively charged regions of water… forms H3O+ (hydronium ions)
water is polar (H+ forms a bond at one of O’s lone pairs)
what do we call H+ also
protons since its made of one proton and nothing else
is
HCl (g) → H+ (aq) + Cl- (aq)
the true ending? is it fully done?
no, bc H+ forms H3O+ with water so… its really:
HCl (g) + H2O (l) → H3O+ (aq) + Cl- (aq)
when do we need to add water as a part of an reactant?
whenever H+ will be one of the products in the “original”
bc if H+ will be one of the products… it will react with water !!
Bronsted-Lowry Theory
- consider what
acid
base
-not all bases contain OH-
-consider eqb equations
acids: proton donors (give up H+)
base: proton receives (receive H+)
hydrolysis reactions def
eqb eqs involving acid or base reacting with water
acids react w/ water to produce H3O+
bases react w/ water to produce OH-
define:
monoprotic acids
diprotic acids
polyprotic acids
monoprotic acids = can donate 1 proton
diprotic acids = can donate up to 2 protons
polyprotic acids = can donate >1 protons
amphiprotic substances def
eg
species that can act as either acid or base depending on the circumstance
water and polyprotic acids that have lost at least 1 proton (at least one H and at least one negative charge)
eg. HCO3 -
on both sides of equilibrium eq will have what
-will have both acid + base on both sides
they will switch as which is which bc eqb
-overall charge is same
define:
conjugate acid + base
conjugate acid
conjugate base
-pair of chemical species that are the SAME EXCEPT for 1 proton
conjugate acid = the one with the extra proton (protonated)
conjugate base = the one without the extra proton (non-protonated)
how to tell if species are ONLY ACIDS or ONLY BASES
egs
HCl
CN-
-COOH
HCl : has proton to donate but no neg charge to gain a proton = ACID ONLY
CN- : has a negative charge to gain a proton but no proton to donate = BASE ONLY
- carboxylic acids, organic acids that end in -COOH: has proton to donate but no neg charge to gain a proton = ACID ONLY
organic acids that end in -COOH
what are they
ACID ONLY bc no negative charge to gain a proton
each eqb reaction has 2 different conjugate pairs
eg. SO3 2- + H2O ⇌ HSO3 - + OH-
identify which acid which base
identify the two different conjugate pairs
SO3 2- + H2O ⇌ HSO3 - + OH-
B A ⇌ A B
one: SO3 2- & HSO3-
two: H2O & OH-
strong and weak acids and bases def
SA + SB
WA + WB
SA + SB = fully ionize/ionize 100%, one-way arrow
WA + WB = do not fully ionize/ionize <100%, eqb arrow
notes about Nitrogen about acid or base
-NH2 or -NH is BASE ONLY
how to tell if NH3 is the acid or base at start..
*note that N is not stable with two lone pairs
NH3 (one lone pair) cannot be acid and turn into NH2-
strong vs weak is NOT SAME as concentrated vs dilute
eg. 0.0001M HNO3 vs. 10M HF
0.0001M HNO3: dilute bc small [ ]; strong bc one-way arrow
10M HF: concentrated because big [ ]; weak bc eqb arrow
-acids produce what in solution
-what happens if the acid is really strong
-produce H3O+ in sol.
-the stronger (eqb arrow; top of arrow) the acid, the greater [H3O+]
-bases produce what in solution
-what happens if the base is really strong
-produces OH- in sol.
-the stronger (eqb arrow; bottom of arrow) the base, the greater [OH-]
spectator ions
who
exceptions
the 5 “bases” at the top of table are conjugate bases of strong acids
but too weak to act as bases = spectators
HSO4 -: can’t act as a base since conjugate base of strong acid BUT also a weak acid = NOT spectator
exceptions: if also a weak acid
-strongest acid
-how is acid strength measured
strongest acid is H3O+ ; all strong acids ionize completely to form H3O+
acid strength is a measure of [H3O+]
Cl- is what
conjugate base of strong acid (one way arrow)
spectator ion since its only that ^
levelling effect def
all strong acids and strong bases are EQUALLY strong (all ionize 100%… cant be more than that)
salts that produce O2- (metal oxides) are
eg?
metal oxides are strong bases (makes O2-)
eg. CaO
strongest base is ____ + forms from what in water
strongest base is OH-
formed from O2- or NH- in water
NH- + H2O → NH3 + OH-
O2- + H2O → 2OH-
how to find [OH-] in other species
eg. in 4.0M Na2O
two ways
multi step way
Na2O → 2Na + O2-
O2- + H2O → 2 OH-
= [OH-] = 8.0M
adding up (net eq?) way
Na2O → 2Na + O2-
O2- + H2O → 2 OH-
- + -—-———————————-
Na2O + H2O → 2NaOH
= [OH-] = [NaOH] = 8.0M
![<p>two ways</p><ol><li><p>multi step way</p><p>Na<sub>2</sub>O → 2Na + O<sup>2-</sup></p><p>O<sup>2-</sup> + H2O → 2 OH- </p><p>= [OH-] = 8.0M </p><p> </p></li><li><p>adding up (net eq?) way </p><p>Na<sub>2</sub>O → 2Na + O<sup>2-</sup></p><p>O<sup>2-</sup> + H2O → 2 OH-</p></li></ol><p>- + -—-———————————-</p><p>Na<sub>2</sub>O + H<sub>2</sub>O → 2NaOH</p><p>= [OH-] = [NaOH] = 8.0M </p><p></p>](https://knowt-user-attachments.s3.amazonaws.com/ce1c2215-5d23-4ec8-8c70-8ba53e2b7a33.png)
how do we know if base needs adding water
if it forms O2- which can’t be stable on own
how to decide which of amphiprotic reactants are acting as acid and base
eg. H2C6H5O7 + HPO4 ⇌ HC6H5O7 + H2PO4
-determining the act as acid: whichever is the STRONGER weak acid (high on arrow; eqb arrow) acts as the acid/donates H+
-the other acts as base
eg. H2C6H5O7 + HPO4 ⇌ HC6H5O7 + H2PO4
since H2C6H5O7 is higher on arrow/stronger weak acid… will donate H+
how to determine if reactants or products are favoured
eg. H2C6H5O7 + HPO4 ⇌ HC6H5O7 + H2PO4
-compare the 2 weak acids in the reactions (will be on opposite sides)
-the STRONGER (look at arrow) weak acid will ionize more therefore pushing it to other way more ∴ other side w/ weaker weak acid is favoured
eg. H2C6H5O7 + HPO4 ⇌ HC6H5O7 + H2PO4
since H2C6H5O7 is higher on list/stronger weak acid than H2PO4… its stronger/push more… favours products (think like make more)
when products favoured, more [ ] of those are made
for a reaction between strong base and strong acid.. whats the net ionic eq
H+ + OH- → H2O
-bc water is the new substance formed
-the salt is aq so not in net eq
kw
what is it
formula
value at 25* or room temp
equilibrium constant for water
Kw = [H3O+] [OH-]
1.00 × 10-14
since Kw is very small, what does this tell us
-water does not ionize to a large extent
not a lot is not made?
-concentration of [H3O+] and [OH-] in pure water is very small + always equal
think that [H3O+] and [OH-] concentration = 1.00 × 10-7
what happens to value of Kw if at diff temp
Kw will be diff since temp. dependent
therefore [ ] of H3O+ and OH- will be diff too
what happens if strong acid or strong base added to water
eg. HCl + H2O → H3O+ + Cl-
will impact ionization of water eq
2H2O ⇌ H3O+ + OH-
eq scenario:
adding strong acid (adding H3O+) ∴ shift left
∴ most of OH- will be consumed during shift BUT NOT ALL
*see graph
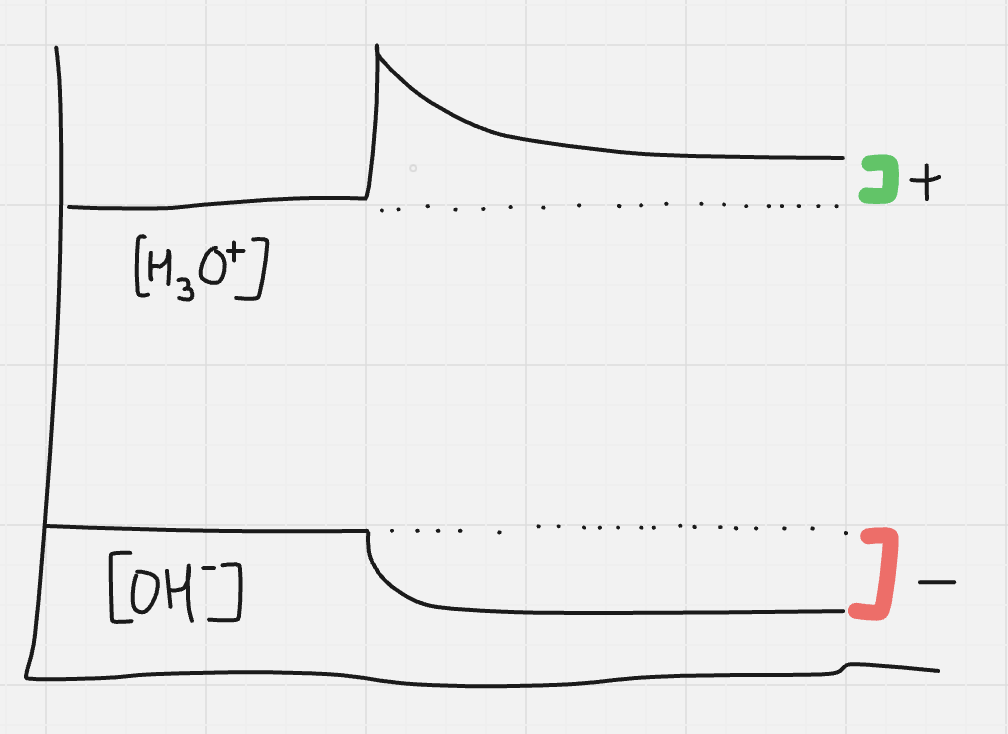
will all of OH- or H3O+ be consumed during shifts from adding strong acid or strong base
NO.
will always be some OH- present in acid sol.
will always be some H3O+ present in basic sol.
since weak acids + weak bases don’t ionize 100%… they__-
-form eqb ∴ can define an eqb expression
this is Keq expressions for behaviour of acid and base in water
-larger the value of Ka..
-larger the value of Kb
-Ka and Kb tells us what
-the MORE the weak acid ionizes ∴ stronger the weak acid
-the MORE the weak base ionizes ∴ stronger the weak base
-Ka/Kb is the measure of acid/base STRENGTH for WEAK acids/bases
is there Ka/Kb for strong acids/base ?
no bc strong acid/base fully ionize (one-way arrow) ∴ don’t have eqb
Kw formulas
Kw = [H3O+] [OH-]
Kw = (Ka)(Kb)
since Kb not in table… how to find
eg. find Kb for HCO3-
use Kw = (Ka)(Kb)
HCO3 + H2O ⇌ H2CO + OH-
Kb = (Kw)/Ka(of base’s conjugate acid/on product side)
Kb = Kw / Ka(of H2CO)
pH neutral at ___ (what number) and at ____ (what temp.)
7 at 25*
pH formula
pOH formula
together formula
[H3O+]
[OH-]
pH = -log[H3O+]
pOH = -log[OH-]
pKw = pH + pOH
[H3O+] = 10-pH
[OH-] = 10-pOH
if need to find pH but from eq of a base
find [OH-]
use Kw =[H3O+][OH-] to find [H3O+]
pH = -log[H3O+]
sf for pH / pOH
number of decimal places is the sf
value of pKw at 25*
14.000 (3 sf)
why is pKw = pOH + pH
Kw = [H3O+][OH-]
log both sides
logKw = log[H3O+][OH-]
*multi log rule = logAB = logA + logB
-logKw = -log[H3O+] + -log[OH-]
pkW = pH + pOH
word problems of making solutions of a certain pH
eg what mass of Sr(OH)2 is needed to make 1.25L of sol. with pH =13.30
eg. what vol of water should be added to 1.00L of HCl with pH=2.25 to increase pH to 2.75
eg1.
find equivalent pOH since given is a base
find [OH-] needed
dissociation eq to find how much [ ] of OH- needed within given Sr(OH)2
mol ratio to find mass (start w/ 1.25L)
eg2.
find initial [H3O+]
find final needed [H3O+]
find final vol will have (sol.) by dilution (M2V2 = M1V1)
subtract initial vol of sol. from final vol will have of sol. = vol. of water needed to be added
when do we know if [H3O+] and [OH-] change equally or not
depends on scenario…
if in pure water only and there’s a temp. change… then will change equally
if add in an acid OR base… then only that [H3O+] or [OH-] will increase and then consumed (unequally)