structure and properties of diamond
1/4
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
5 Terms
diamond is an allotrope of carbon - what is an allotrope?
Allotropes are structures that have the same chemical properties but different physical properties due to differences in structure and bonding.
how many covalent bonds should carbon form to gain a full outer shell?
because carbon is in group 4, it will need to make four covalent bonds to gain a full outer shell
how many bonds does carbon form in diamond?
4 - Each carbon is bonded to four other carbons
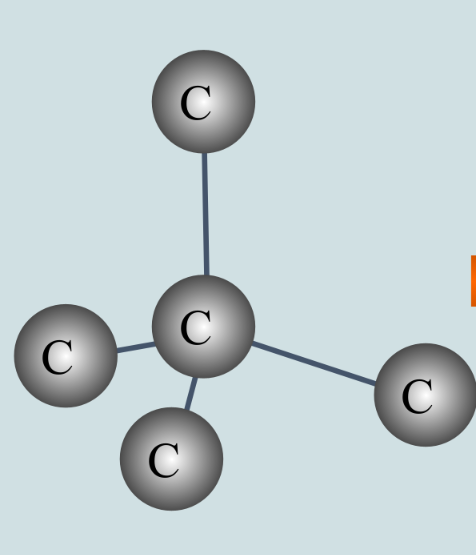
what is true about diamond’s hardness and strength and why is this?
diamond is very hard and strong
because the giant covalent structure in diamond is so rigid, it is resistant to scratches and keeps its shape even when a force is applied to it
diamond is the hardest natural structure
what is true about diamond’s ability to conduct electricity and why is this?
diamond cannot conduct electricity
because each carbon forms 4 covalent bonds, there are no delocalised electrons
because of this, no charge can be carried through the structure so no electricity can be conducted