MedChem Exam 4: Extraction Chromatography
1/86
Earn XP
Description and Tags
HPLC, GC, Extraction
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
87 Terms
What percent of drug can you extract in a single extraction if the partition coefficient is 5 and you have 5 ml of patient plasma and 5 ml of ether? Enter the value as a the nearest whole number without the percent sign.
83%
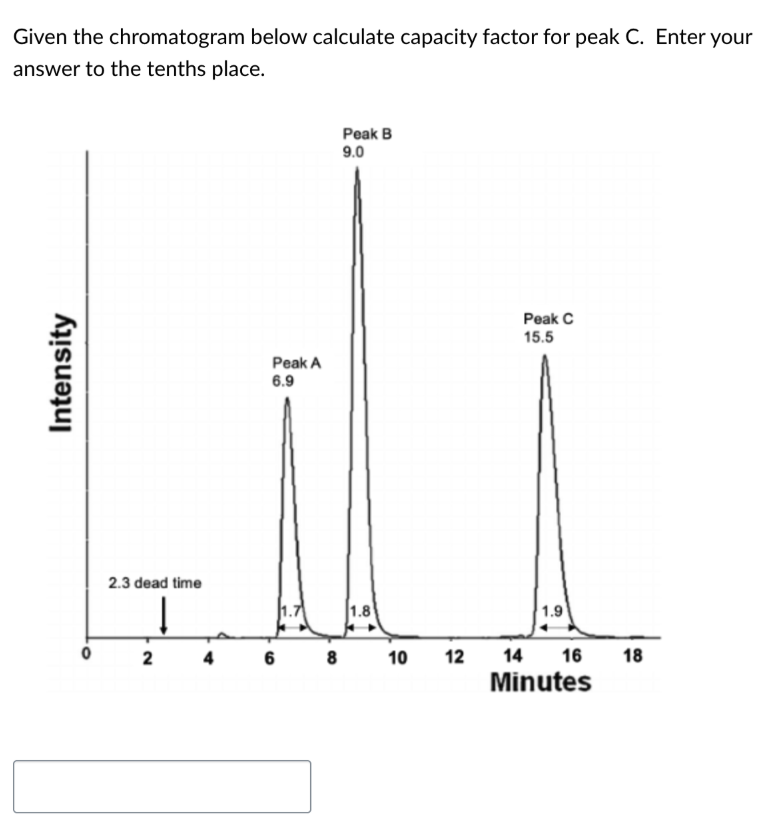
5.7
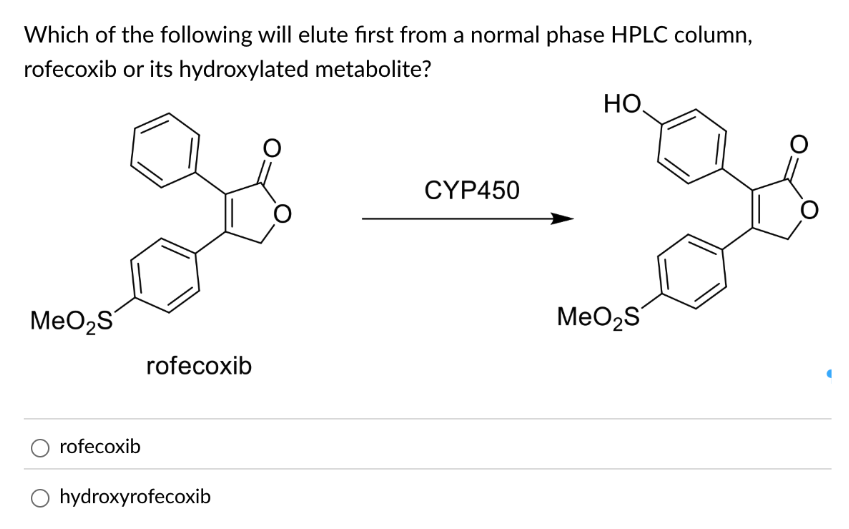
Rofecoxib

Analytes partition between the mobile phase and the stationary phase.
Diazepam
Biological samples are
complex mixtures that have drug+proteins+sugars+lipids+nucleic acid
Steps of sample analysis
Biological samples are collected
Purification: Extraction or Chromatography
Detection: Spectroscopy or Mass Spectrometry
What do we want to achieve through sample purification step?
remove contaminants that interfere with the identification/quantification of the drug or its metabolites
2 goals during sample purification
maximize yield / minimize loss
minimize processing time
Describe 2 different sample purification steps
extraction or back extraction: isolates drug + other organics
chromatography: ideally separates mixture into individual molecules
Name 2 different chromatography methods
HPLC ( High Pressure Liquid Chromatography)
GC (Gas Chromatography)
During Detection step, what do we detect and how?
Detection of small molecule, typically with quantitation
During Liquid-Liquid extraction, what conditions are remain constant?
temperature and pH
What type are divides in two immiscible solvents: aqueous and organic? and what does it represents?
any neutral, non-dissociating chemical species
ratio of the concentration or the partition coefficient (P=[org]/[aq])
Describes the steps of liquid-liquid extraction
aqueous biological sample
add immiscible organic phase (not forming a homogenous mixture when added; often octanol or ether)
shake to increase surface contact (molecules partition by polarity)
isolate target layer
Formula used to calculate the fraction of drug in organic phase
forg= PV/PV+1
P: partition coefficient—> given
V: Volume(org)/ Volume (aq)—> experimentally determined
How do we maximize the yield of liquid liquid extraction? (Hint: 2 different ways)
larger volume of organic phase
multiple extractions: extract multiple times by adding organic solvent (if target drug is dissolved in this solvent) to the remained aqueous solvent
When does multiple extractions are worth it?
when we quantifying something very small
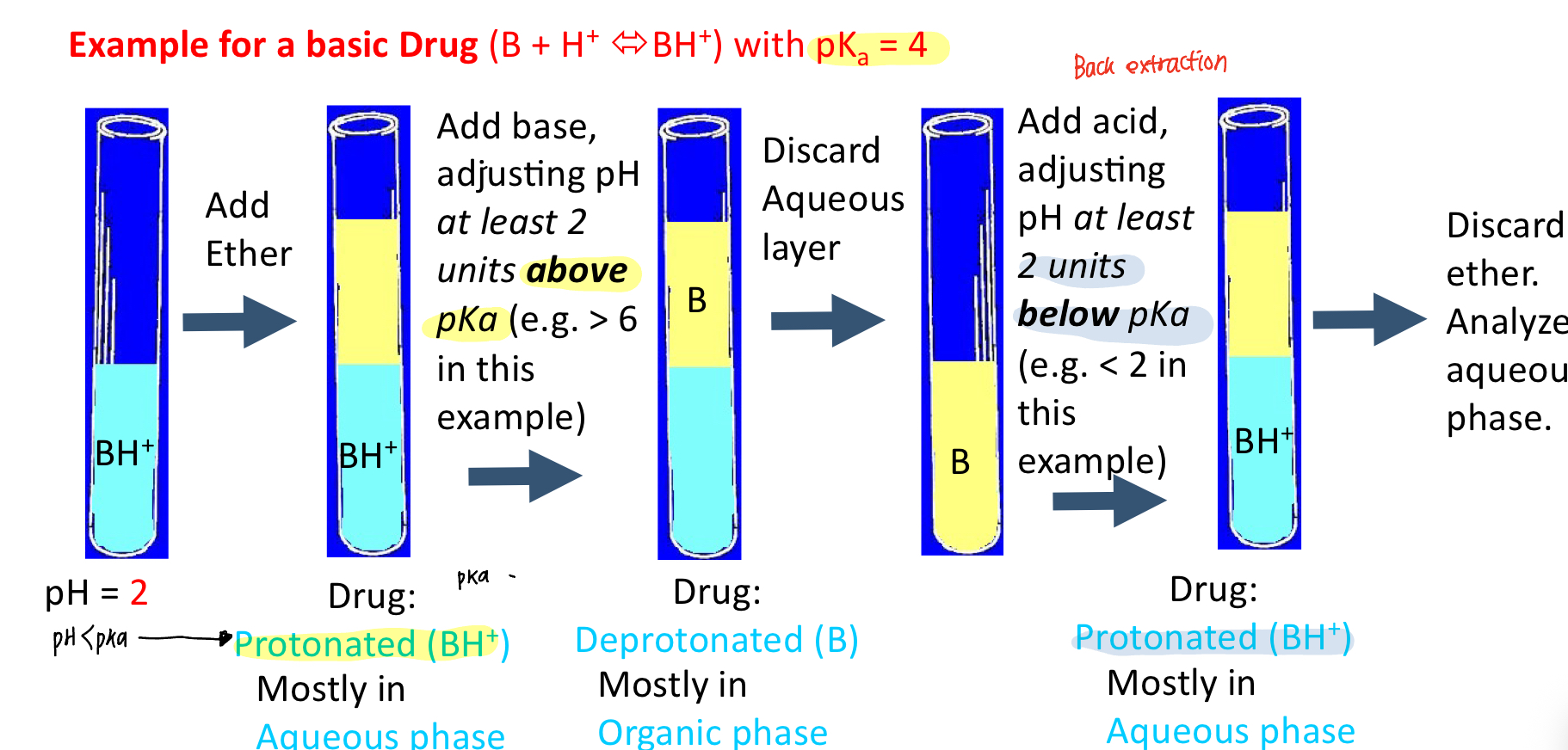
When do we use back extraction method
ionizable compound that can be manipulated by pH
Describe the steps of back extraction (acid drug)
make compound neutral for extraction into an organic solvent —> adding acid makes drug protonated
then make the compound ionized to back extract into aqueous—> adding base makes. drug deprotonated
e.g. drug pka is 4
pKa>pH: HA
pKa<pH: A-
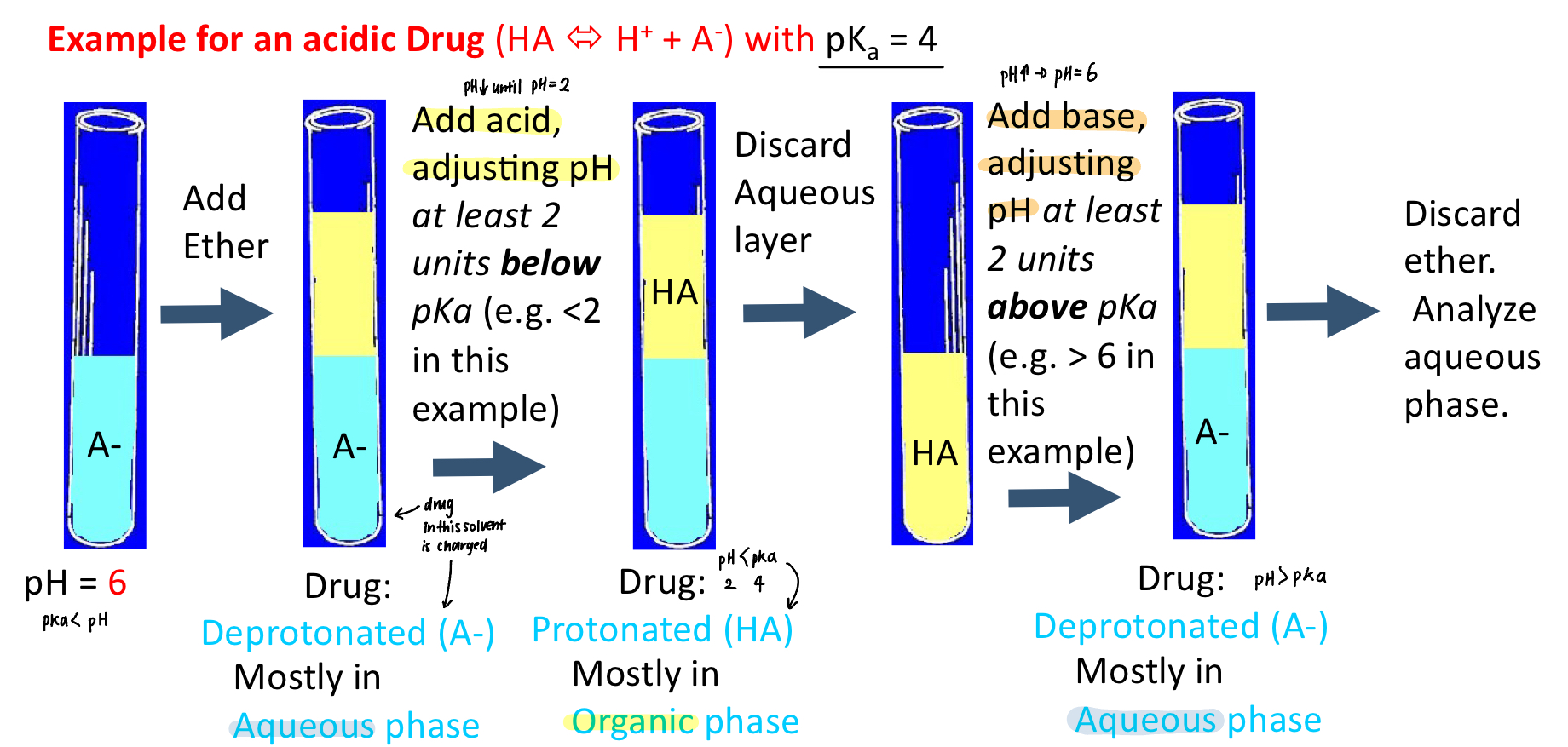
Describe the steps of back extraction (base drug)
make compound neutral for extraction into an organic solvent —> adding base makes drug deprotonated (neutral)
then make the compound ionized to back extract into aqueous—> adding acid makes drug protonated (charged)
e.g. drug pka is 4
pKa>pH: BH+
pKa<pH: B
What is chromatography?
separation of closely related molecules of complex mixtures based on partition coefficient between a mobile phase with sample in either gas or liquid and stationary phase with similar physiochemical characteristics to sample components to be separated
sample that is mixed as liquid goes to
HPLC
Two different methods of mobile phase forced through a stationary phase
column & plannar
Describe the column chromatography using analyte, mobile phase, stationary phase, migration rate
analyte with two or more molecules in the top of the column applied to the stationary phase held in a column by introducing mobile phase
Mobile phase flows through stationary phase
Migration rate of different molecules in analytes differ depending on the time spent/ affinity in stationary phase vs mobile phase
the faster the molecule comes out (when stationary phase is packed in the column)
has good affinity with mobile phase
what happens if a molecule is only attracted to stationary phase in TLC paper
does not move= no elution
what happens if two molecules are both only attracted to the mobile phase in TLC paper
no separation=coelution
we need to identify a _____ where analytes have partial partitioning to _____
happy medium
both phases
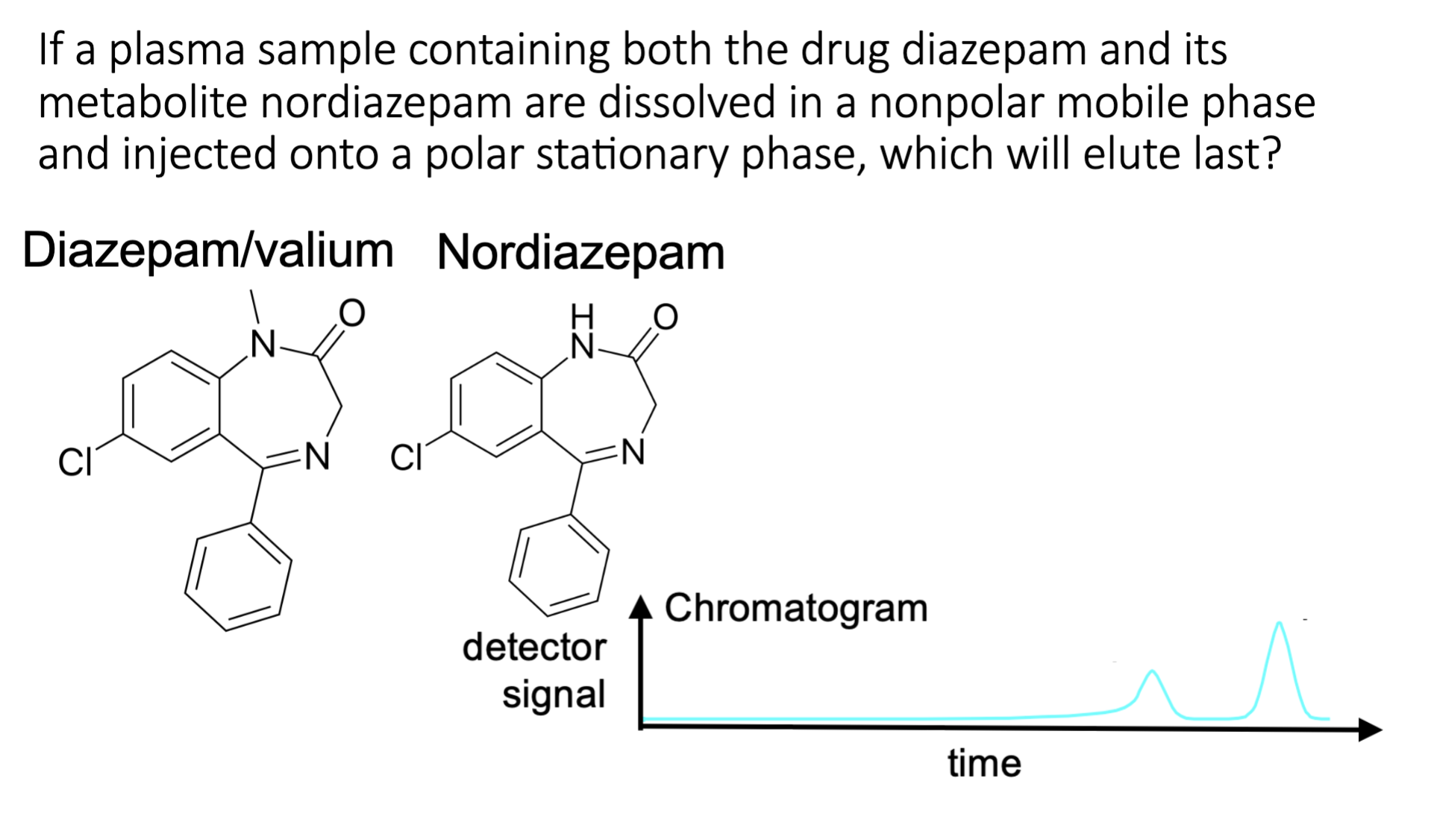
analytes on the column
diazepem is nonpolar
nordiazepam is polar due to NH
mobile phase is nonpolar
interact with diazepem
stationary phase is polar
interact with nordiazepam
Thus, diazepam elute first as it has a better affinity with mobile phase and then nordiazepam.
Overall goal of chromatogram is to have _______ peaks and eluting in reasonably ______
well separated and short time
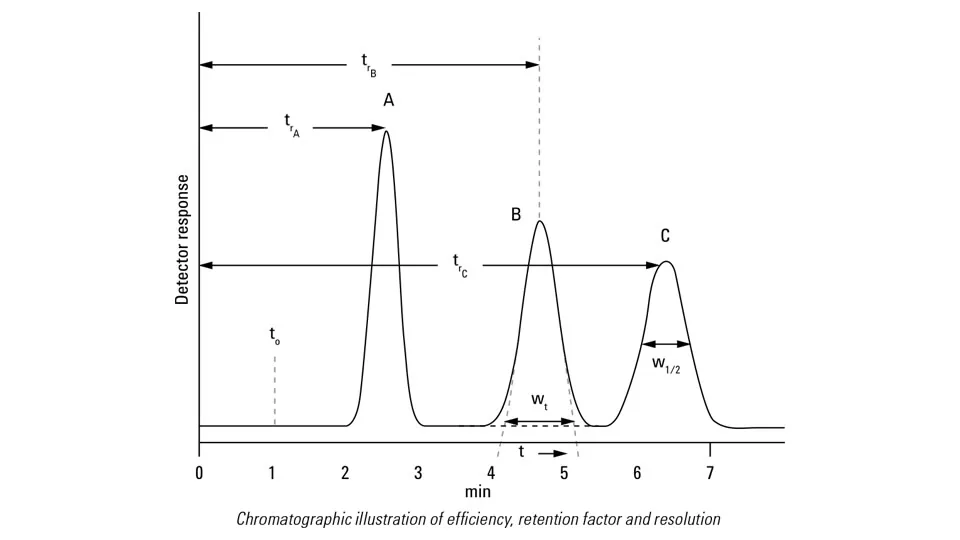
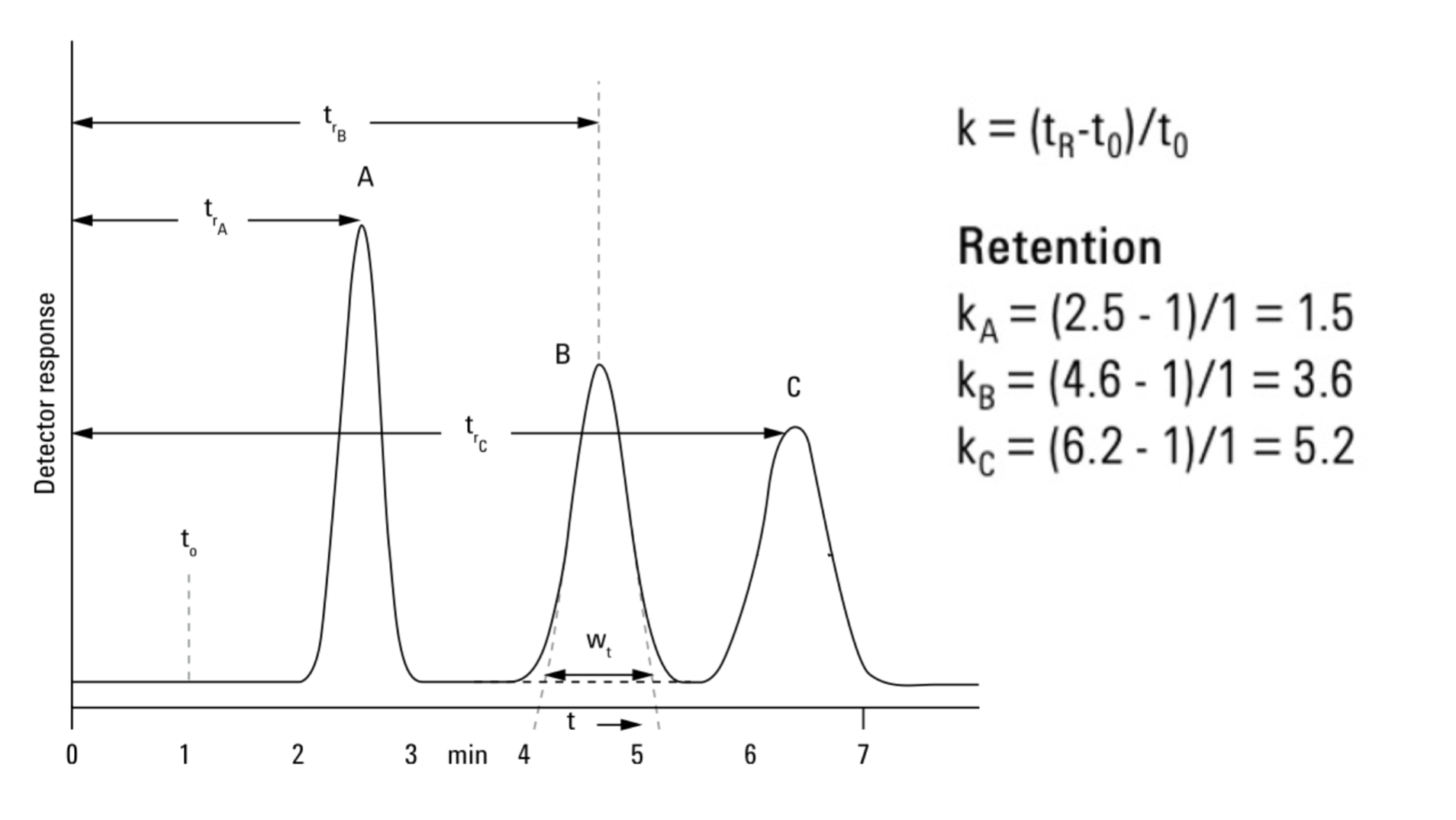
What does each of letters in this graph represents?
t0: void time or dead time —> time for one volume or no volume is going through column; nothing can go faster than this elution time
tr: retention time—> the time elapsed from the moment a sample is injected into a chromatographic system until a specific compound is detected at the end of the column; change depending on the molecules affinity to phases
wt: broader base width—> broader with longer retention time if consistent mobile phase or isocratic is used
Why do we have to short run a chromatography under isocratic mobile phase?
samples that elute later have more wider than the samples that elute earlier, resulting poor sensitivity and bad resolution or detection
What are the factors that affects chromatography? (hint: 3)
Efficiency
Retention factor or capacity factor
Selectivity or separation factor
What dose efficiency represents and used?
how well components in analytes are separated and compare columns
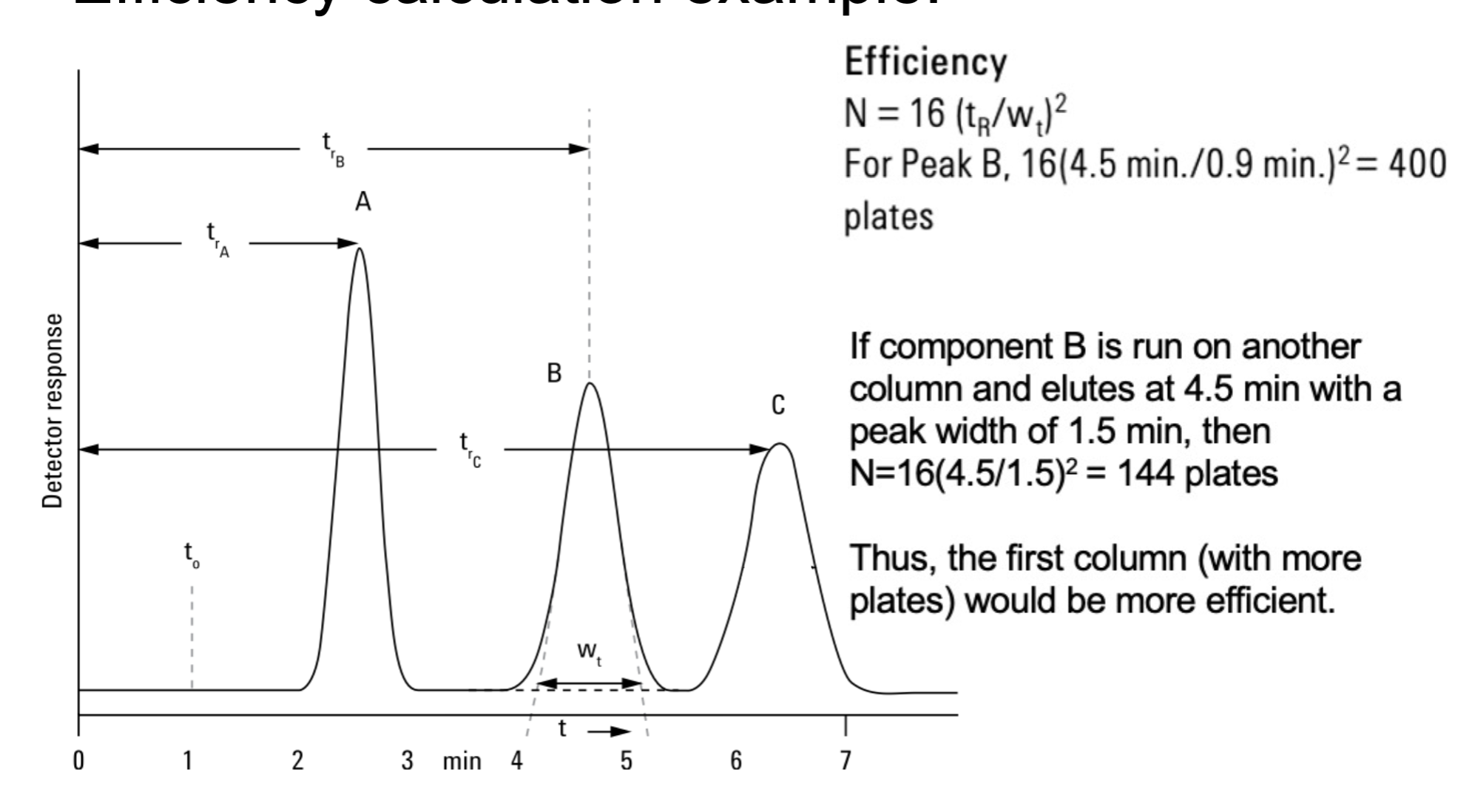
how does efficiency is expressed and its formula?
N and N=16 (tR/Wt)2
N values often (range) with higher number.
High N have a ____ peak at a given retention time than one with lower N
5000-25000
narrower
What are three factors that increase efficiency?
longer column
smaller stationary phase particle size
lower viscosity (fast flow) of mobile phase
How does having
longer column
smaller stationary phase particle size
lower viscosity (fast flow) of mobile phase
contribute to increasing efficiency?
longer column = more plates ( more faster partition between two phases)
smaller size = fast particle diffusion in stationary phase = shorter tr and narrow width
lower viscosity = analytes within mobile phase move faster through stationary phase→ less diffusion over time
What is retention factor or capacity factor (k,k’,k’ A)
formula
the time a molecule resides with stationary phase relatve to the time it partitions in mobile phase
formula: k= (tR-t0) / t0
retention factor is increased by (hint: 2 ways)
selecting stationary phase more similar to analyte while less similar to mobile phase
decrease temperature
what is the optimal value of retention time
having fast or slow retention time can be bad; change depending on the context
>1 at elutes at t0 and <10 (>10: longer run time, peaks widen)
what is selectivity or separation factor (alpha)
formula
measure of the time or distance between two peaks
a= k2 (second peak) / k1 (first peak)
how to increase separation?
changing the mobile phase, stationary phase, running temperature
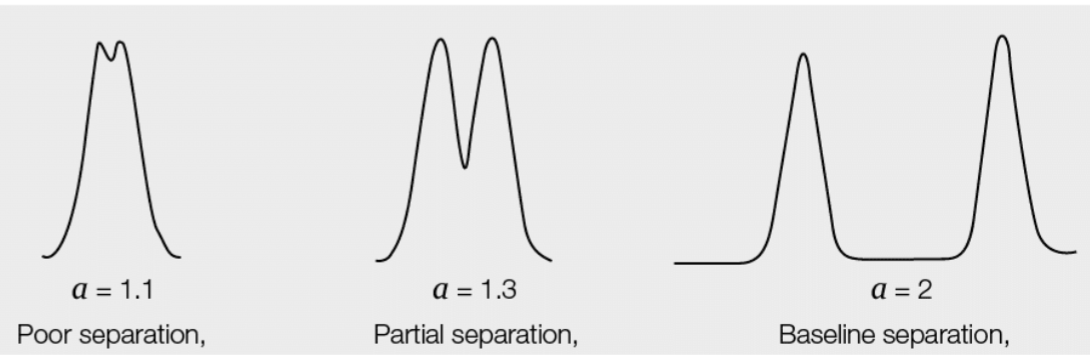
what is ideal value of separation? what happens is separation=1
a=1 the two peaks co-elute(no separation)
>1.6 is ideal
What does efficiency, retention, selectivity contributes?
resolution
what is resolution
formula
ability to separate the peaks considering efficiency, selectivity, and retention
Rs= route(N)/4 (a-1)/a k/(k+1)
what is the minimum R value for a separation to allow quantitation
R=1
what is desirable for rugged methods
what is baseline separation ensuring the most accurate quantitative result
>_ 1.7 and 1.6
resolution can improve by changing any one of the individual parameters. but the most impactful parameter is
selectivity
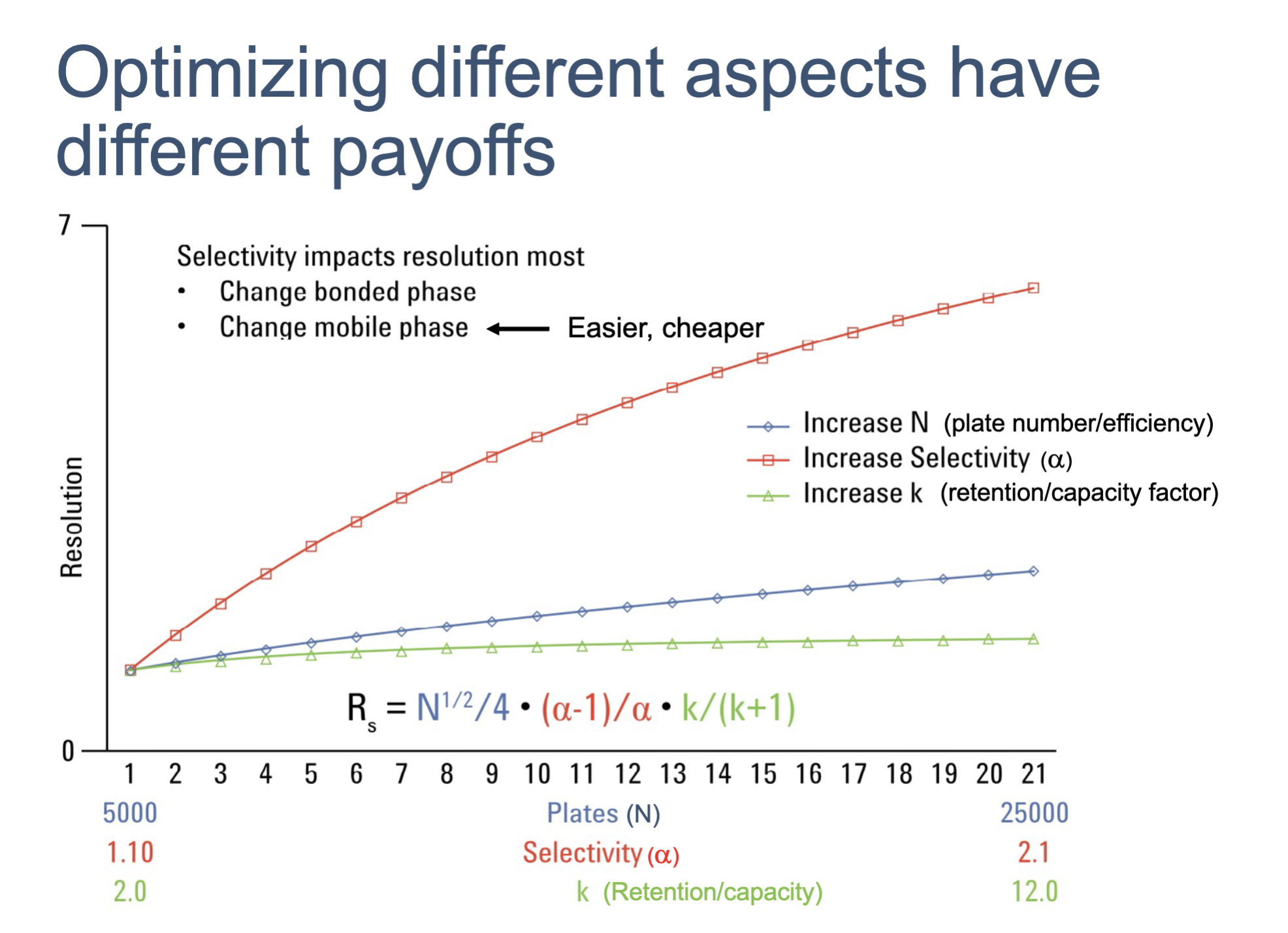
how does selectivity can be changed?
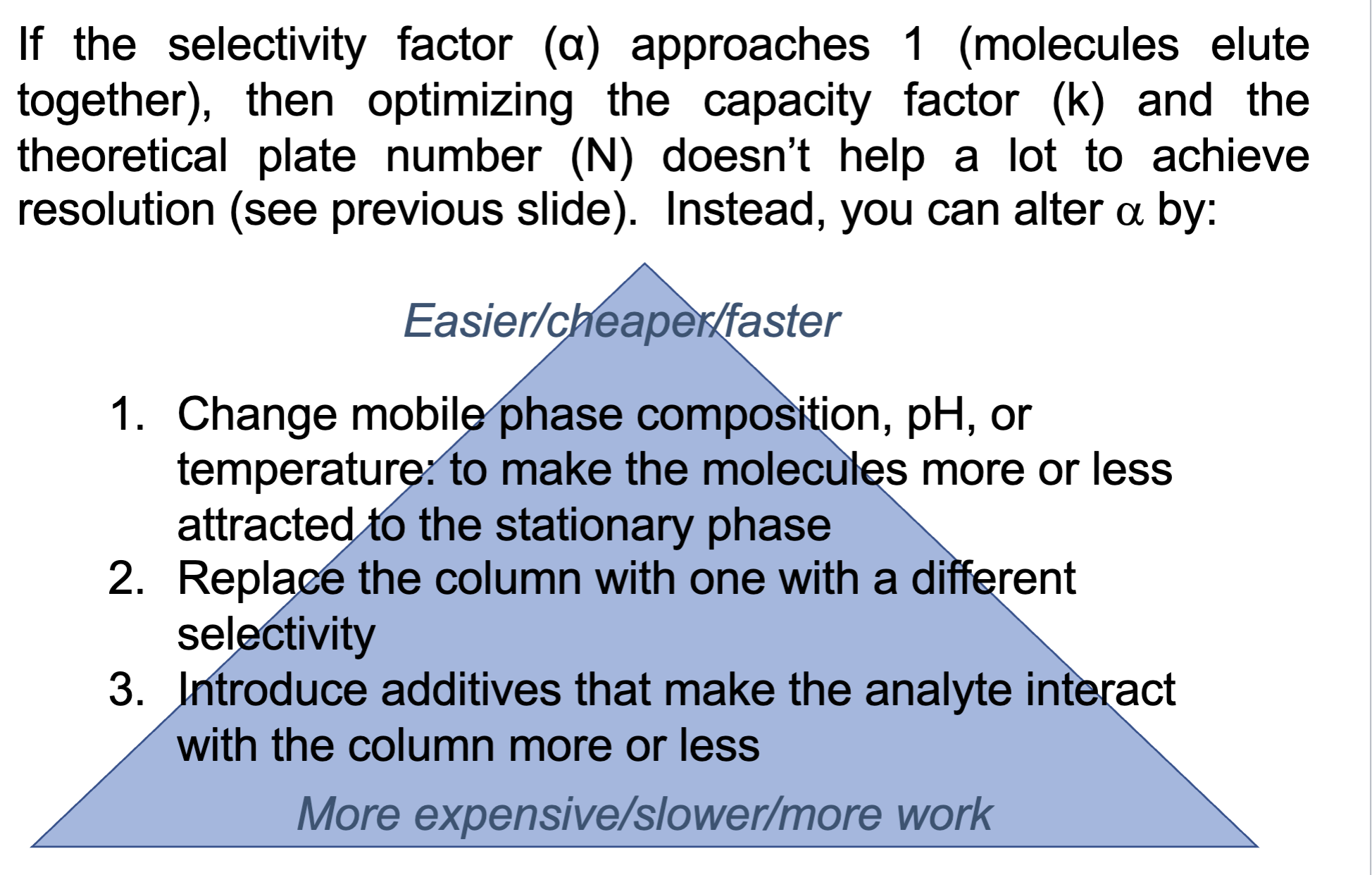
true or false: If the selectivity factor (α) approaches 1 (molecules elute together), then optimizing the capacity factor (k) and the
theoretical plate number (N) doesn’t help a lot to achieve
resolution (see previous slide).
true
two different ways to resolve peaks that had resulted under a constant mobile phase composition (isocratic run) due to mixture being complex:
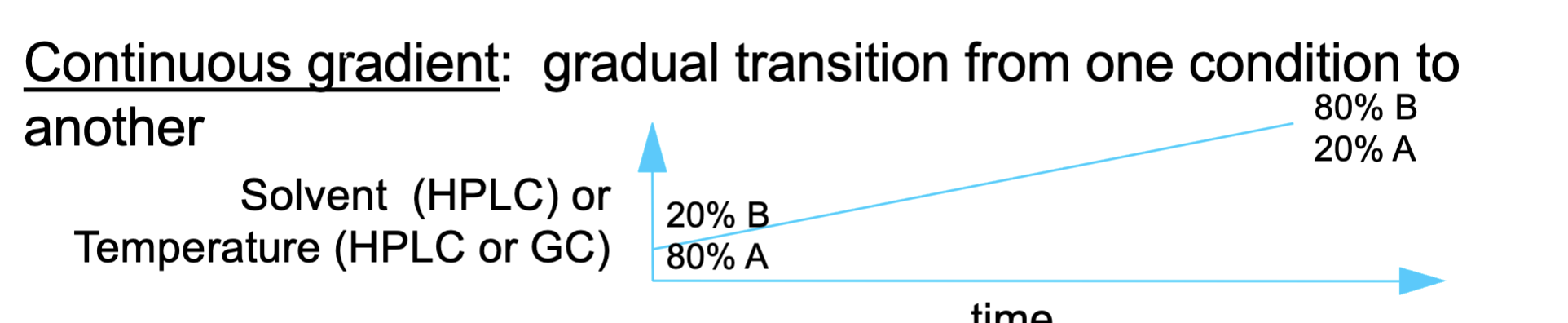
continuous gradient
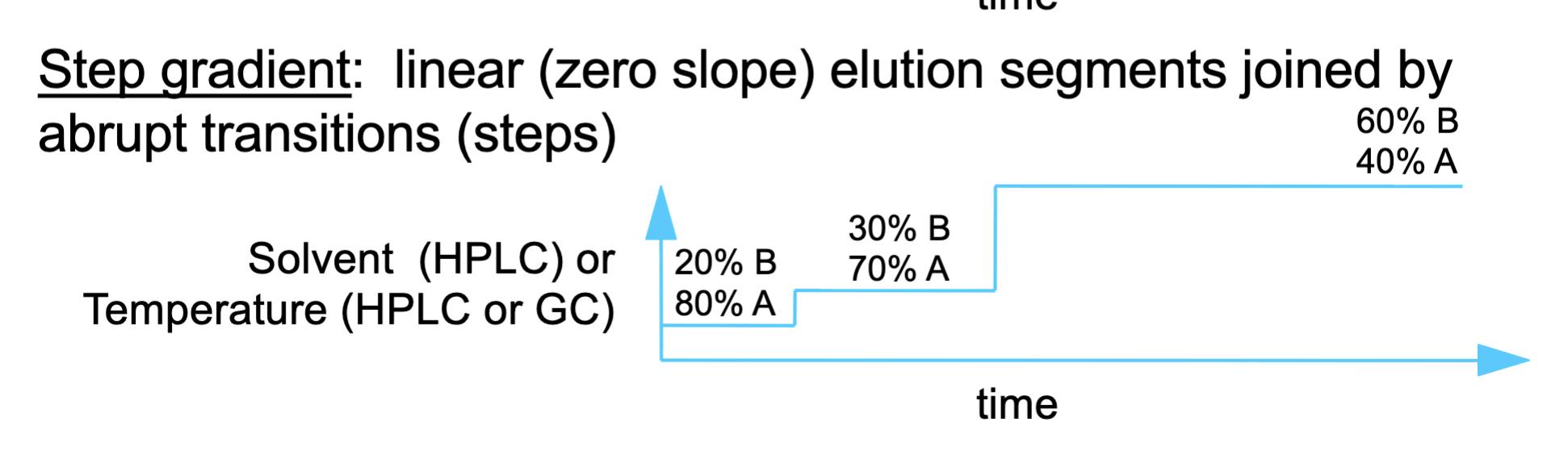
step gradient
continuous gradient means
The mobile phase changes gradually and continuously over time.
step gradient
The mobile phase changes in sudden jumps, not gradually.
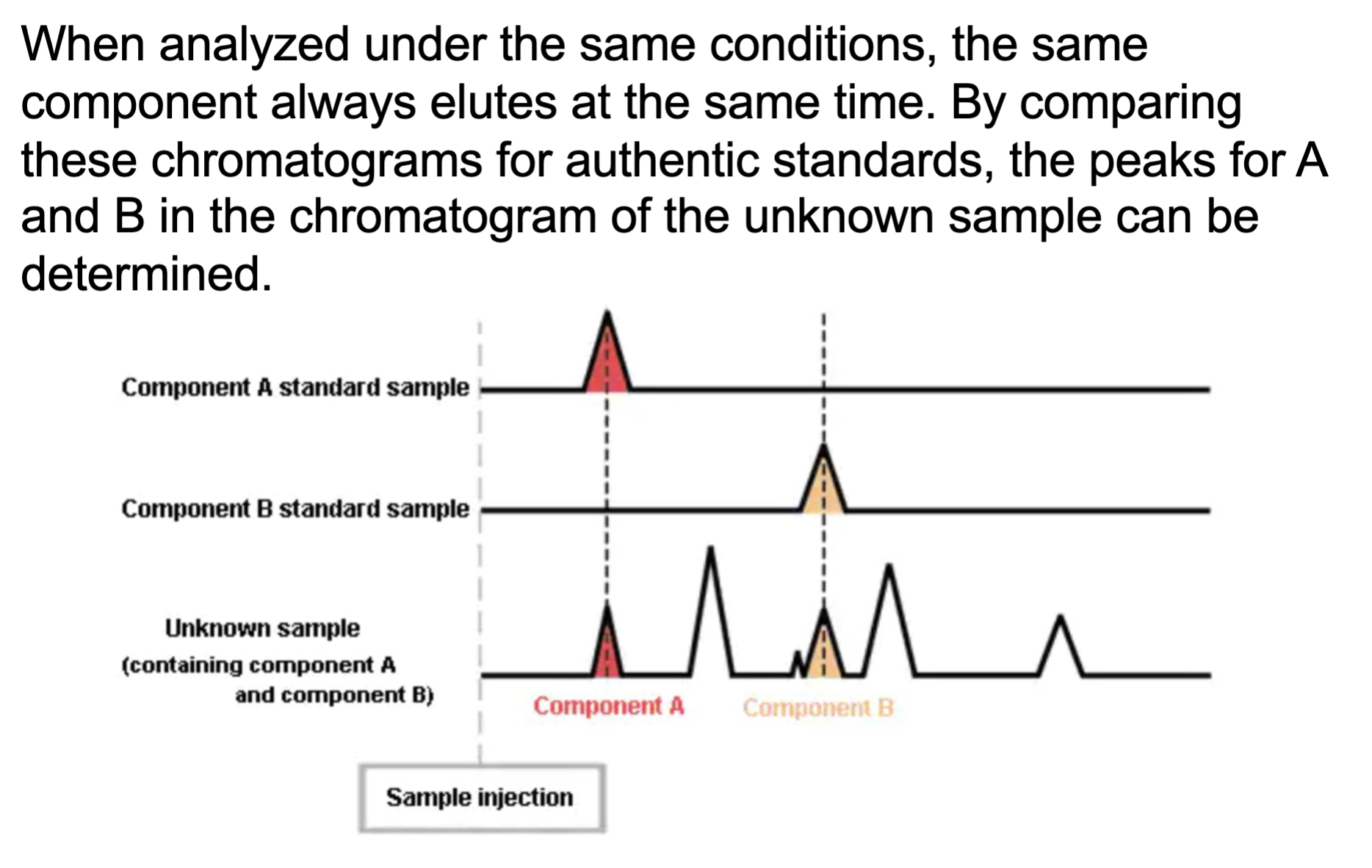
the chromatogram of the unknown samples can be determined by the
authentic standards as the same samples under the same conditions are elutes at the same time
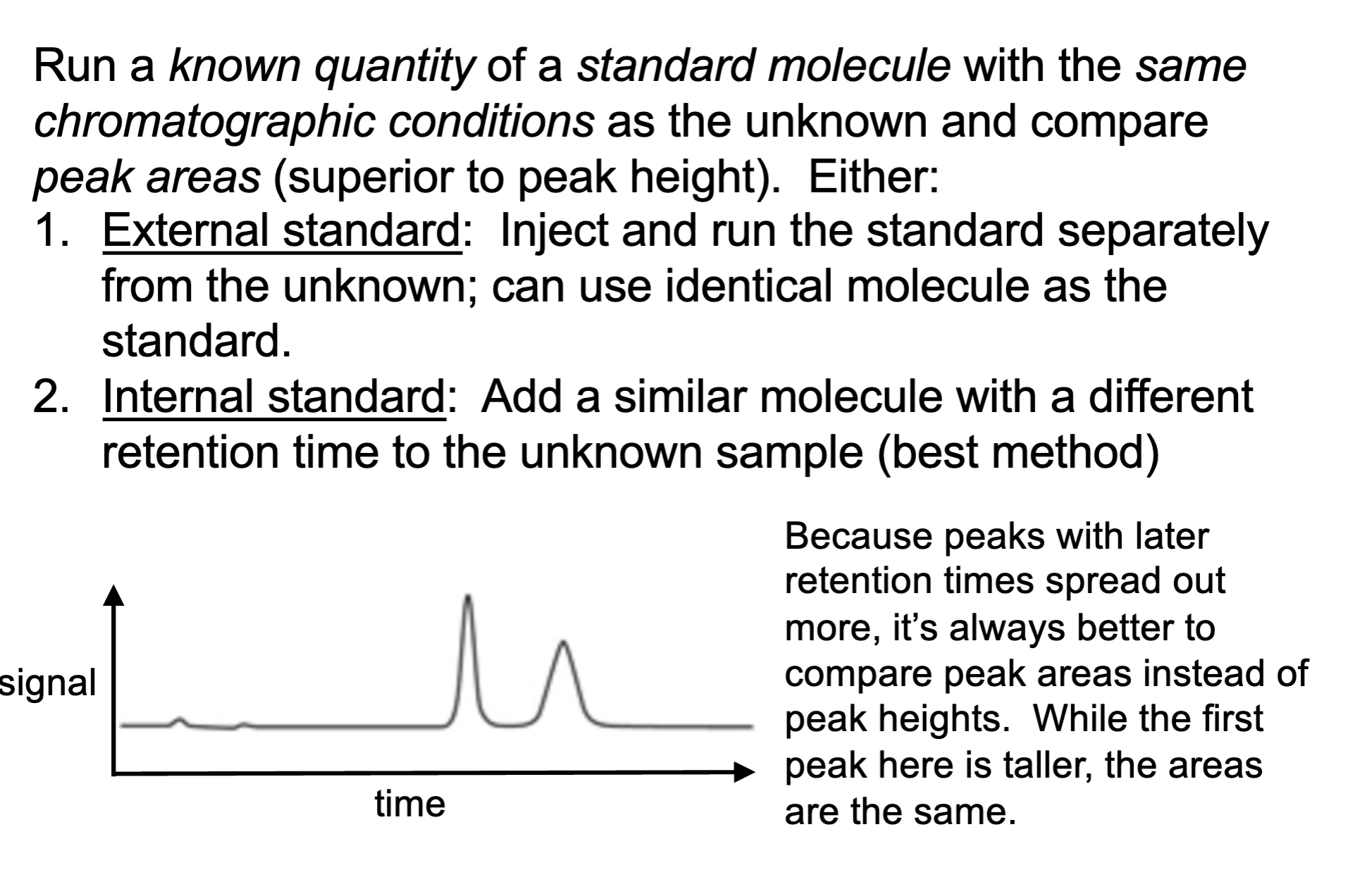
how do we quantity a unknown compound
running a known quantity of a standard molecule with the same chromatographic conditions as the unknown.
when comparing an unknown compound quantity using known stand, we compare ___ than ____
compare the peak areas (superior to peak height)
Why is peak area better than peak height for quantitation?
Late-eluting peaks broaden more and become shorter in height, even if the total amount is the same.
➡ Area stays constant, height does not.
➡ So area gives more accurate quantitation.
what are two types of standard used to compare the unknown standard quantity
external standard and internal standard
what is external standard
explain the process of getting the concentration of unknown
A known quantity of analyte run separately from the unknown.
You prepare multiple standards → measure peak AREA → plot them → make one calibration curve: peak area vs concentration.
Then you inject the unknown → measure its peak area → plug them in the calibration curve found by standards to calculate the concentration
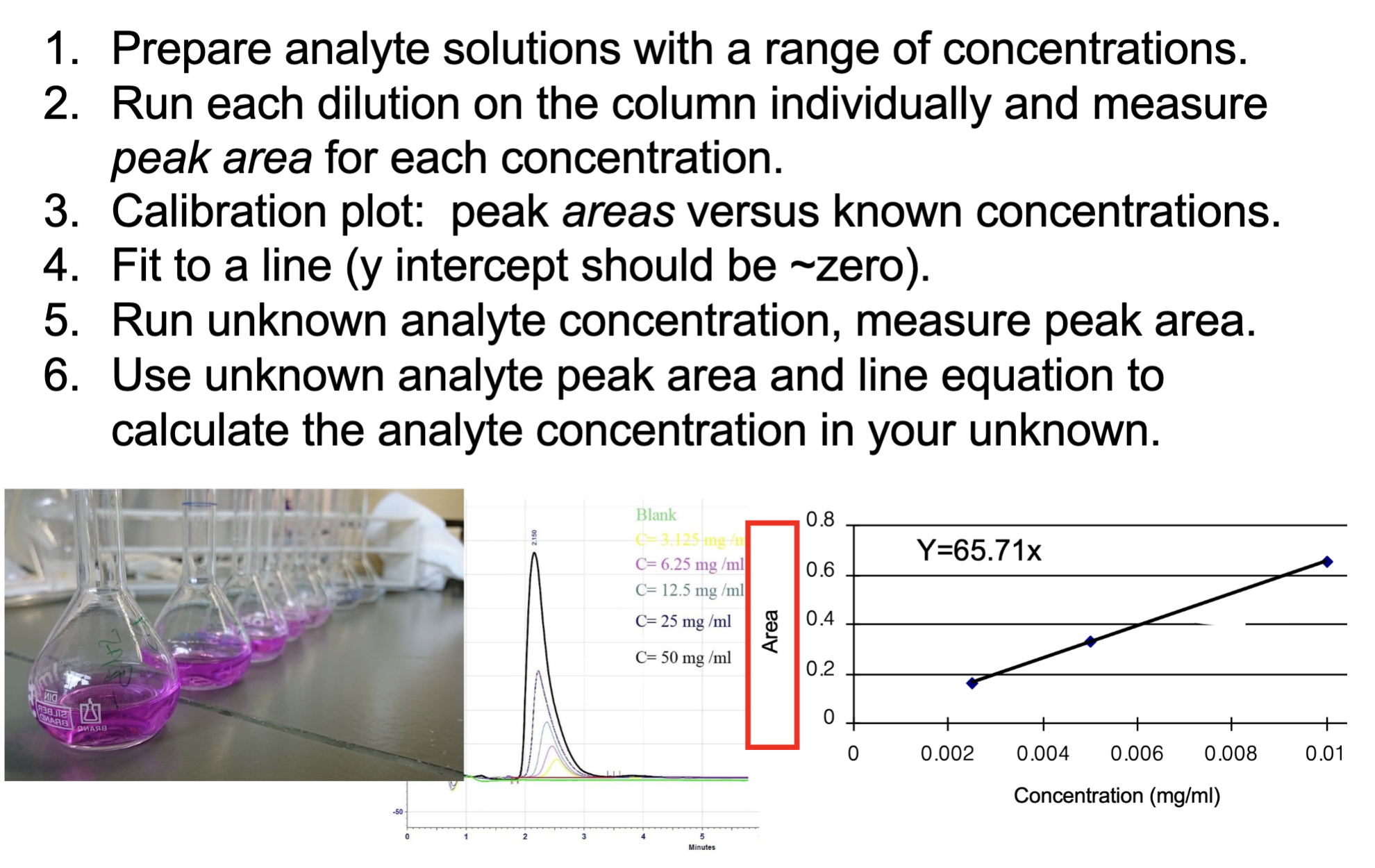
What do you get when you plug unknown peak area into the external standard calibration equation?
The concentration of the analyte in the unknown sample.
What is an internal standard?
Steps of internal standard method?
A known amount of a chemically similar internal standard compound that is added to every external standard and unknown samples.
- use linear fit to calculate the concentration in unknown samples
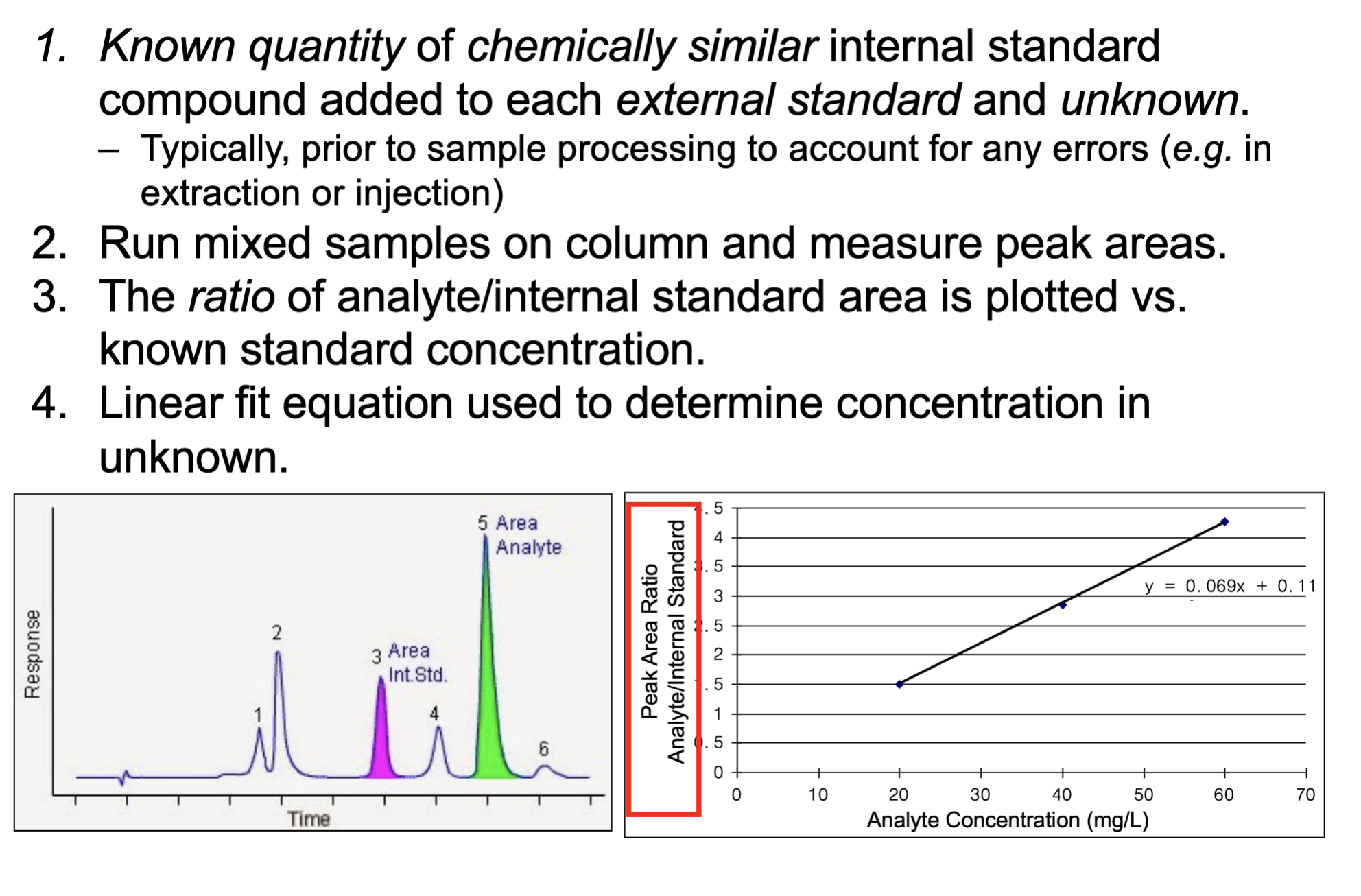
what standard is preferred and why?
internal
Uses the ratio of analyte area / internal standard area, which makes results more accurate as it accounts for anyu sample processing errors.
Two types of chromatography
high pressure liquid chromatography
gas chromatography
high pressure liquid chromatography resolves moleucles in the molecular range of
100-10,000amu or Da
HPLC type depends on _________ to siloxane polymer particles (hint: 3 different FG)
which functional groups added
polar
nonpolar
chiral
Normal phase HPLC has a _____ stationary phase and ____ mobile phase
what are the examples of _____ stationary phase & mobile phase
polar, non-polar
polar
cyano
diol
amino
dimethylamino
non-polar
chloroform
n-hexanes
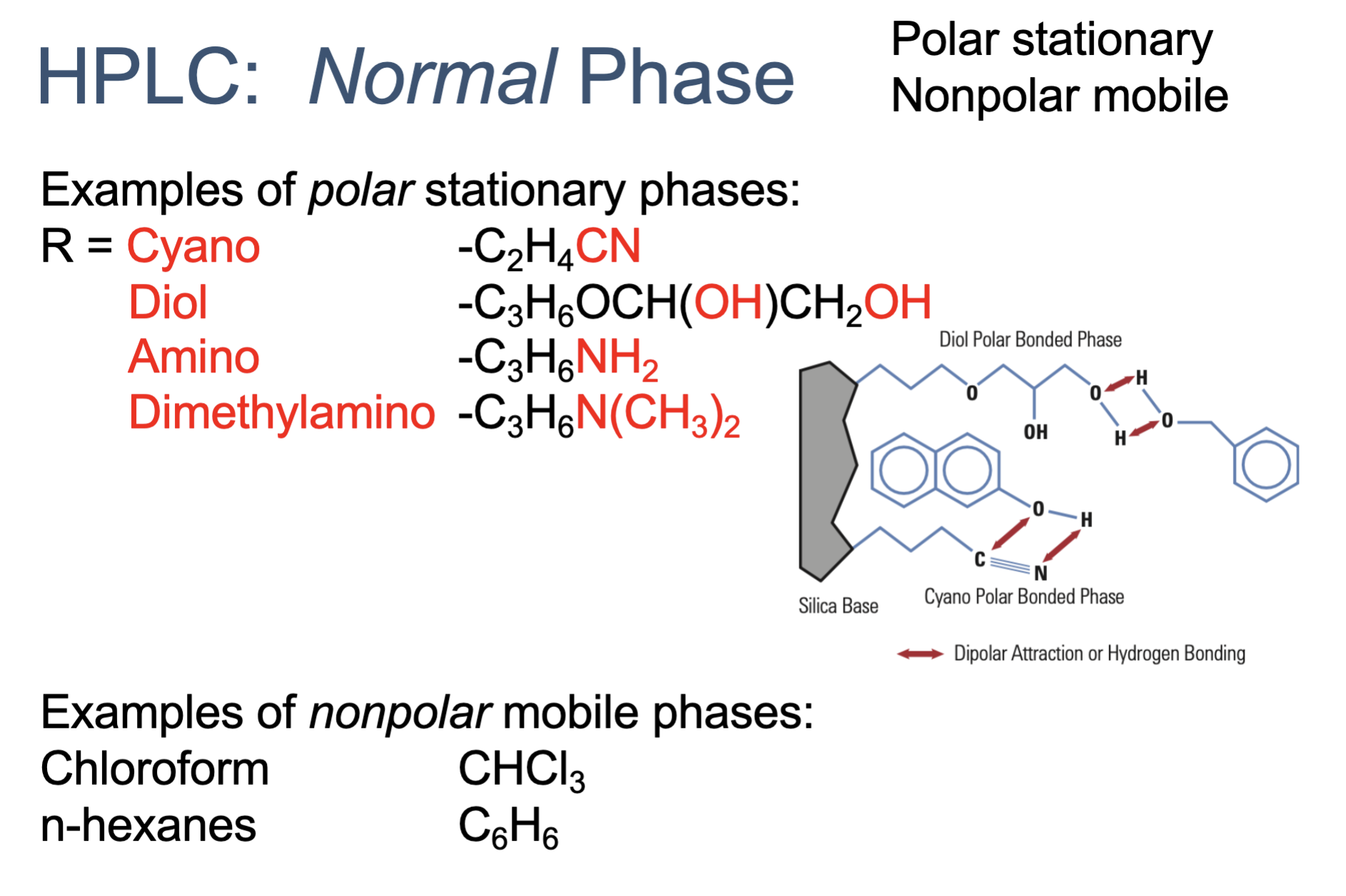
what polarity of molecules elute faster in normal phase HPLC?
nonpolar molecules elute faster
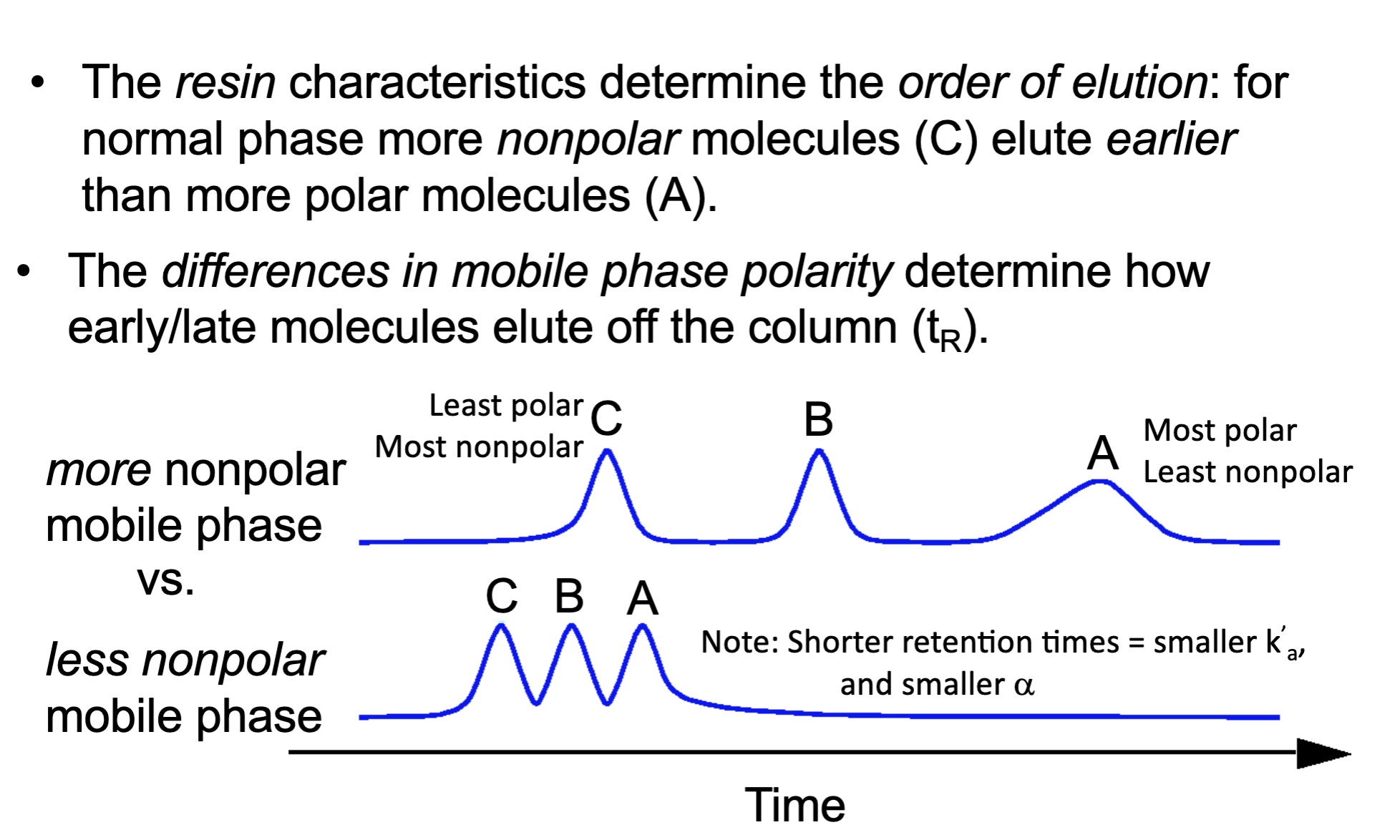
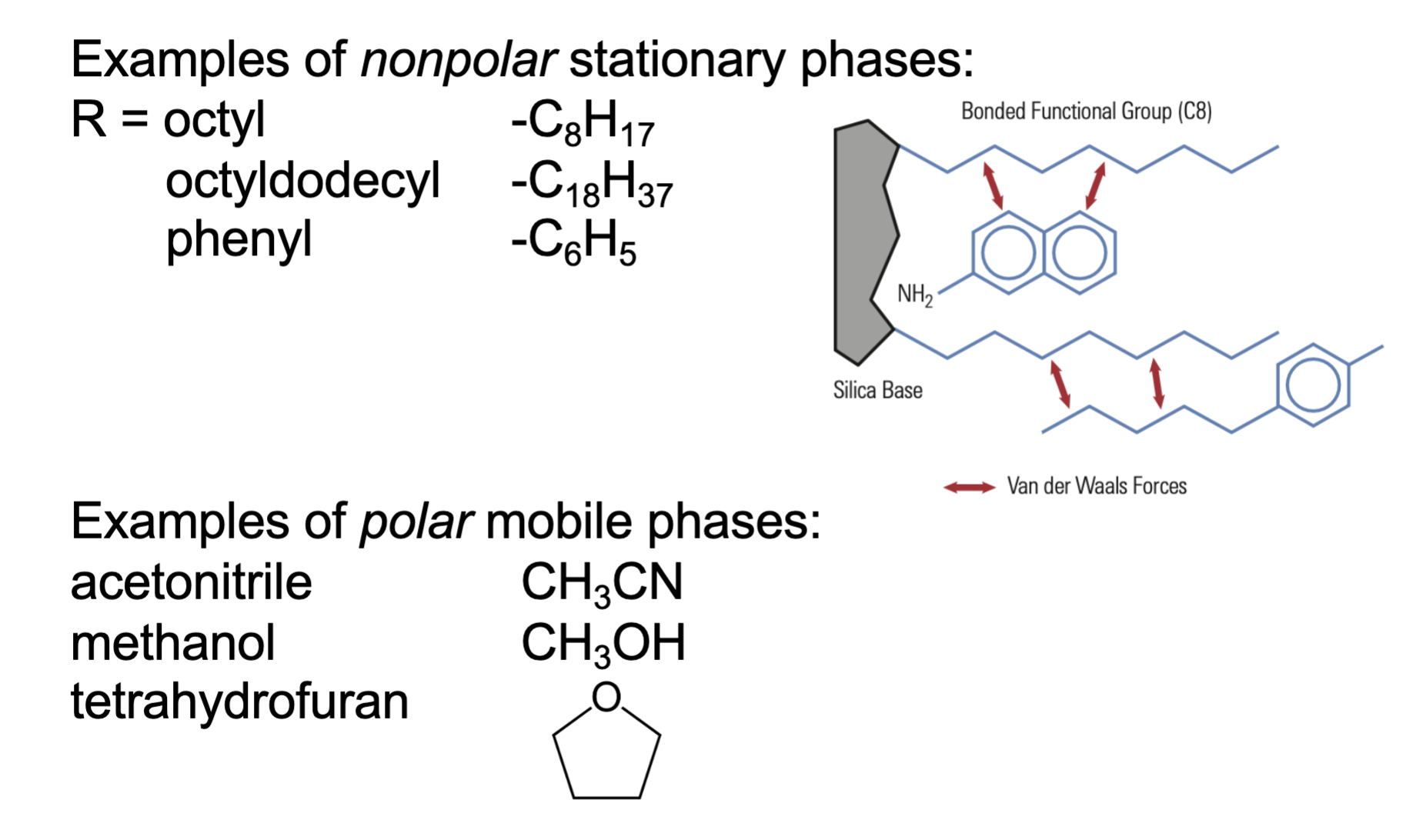
Reverse phase HPLC has a _____ stationary phase and ____ mobile phase
what are the examples of _____ stationary phase & mobile phase
nonpolar, polar
nonpolar
octyl
octyldodecyl
phenyl
polar
acetonitrile
methanol
tetrahydrofuran
what polarity of molecules elute faster in normal phase HPLC?
polar molecules
the differences in _________________ determine how early/late molecules elute off the column (tR)
mobile phase polarity
True or False: Enantiomers cannot be separated by reverse or normal HPLC
True
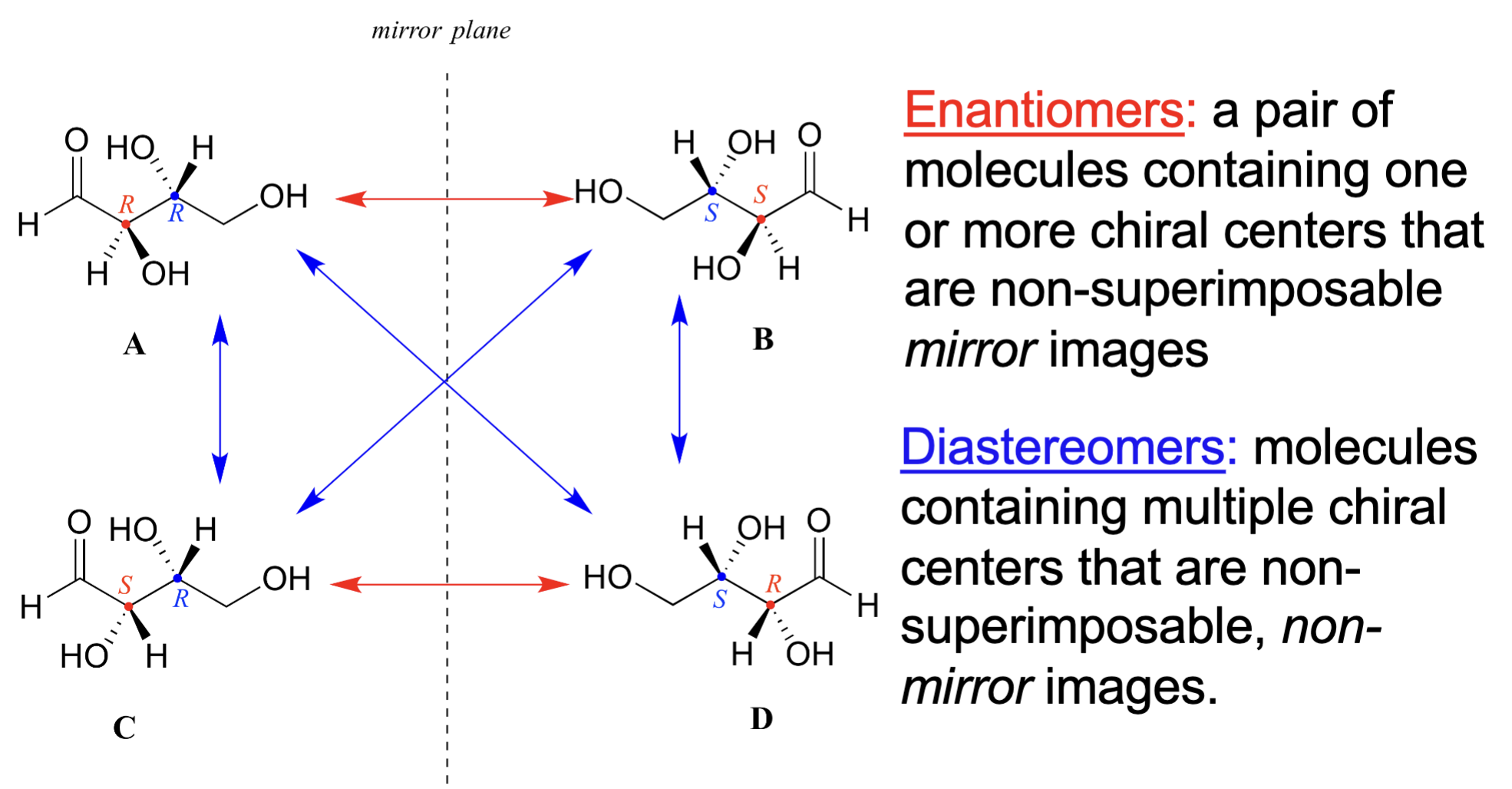
what is enantiomer and diastereomers
enantiomers: non-superimposable mirror images
diastereomer: non-superimposable and non-mirror image
How do we use enantiomer in HPLC
Per-Column Derivatization: Convert Enantiomer to Diastereomers Before HPLC
React Enantiomeric Mixture with One Enantiomer of a Chiral Derivatizing Agent to Form Diastereomers
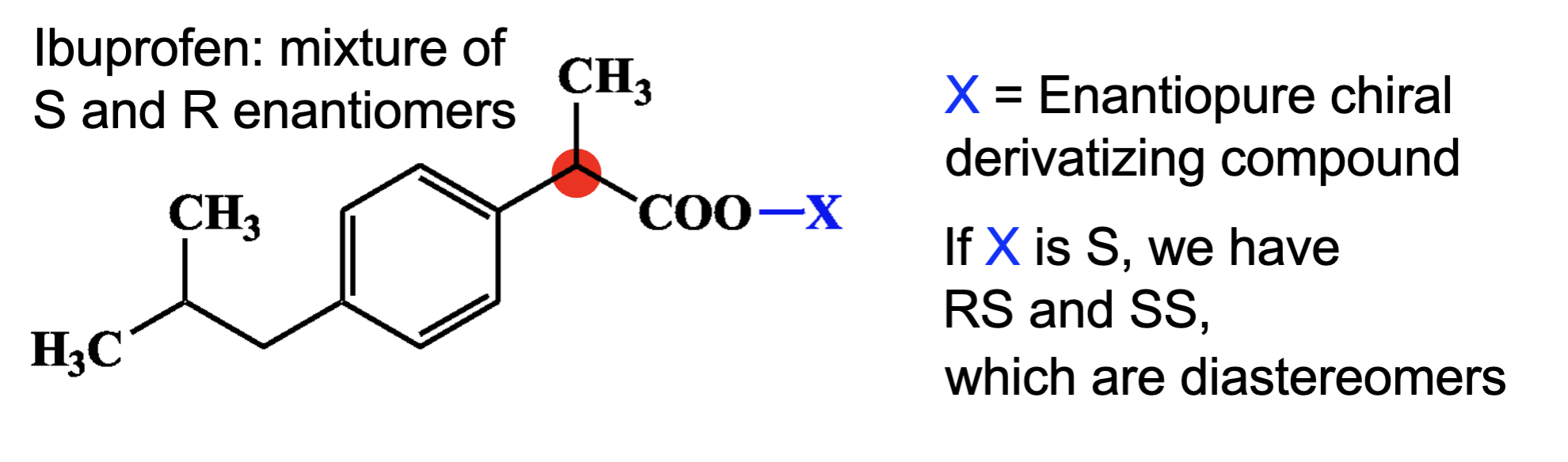
If the mixture of S and R used, what happen after adding enantiopure chiral derivatizing compound?
SS and SR which are diastereomer
what happen if racemic mixture A reacts with racemic derivatizing agent B?
racemic mixture A is S-A and R-A
racemic mixture B is S-B and R-B
S-A/S-B
R-A/S-B
S-A/R-B
R-A/R-B
R-A/S-B & S-A/R-B —> enantiomers
S-A/S-B & R-A/R-B —> enantiomers
Thus, only two peaks are formed and this is reason why we only need one derivatizing agent
What is the advantage & disadvantage of pre-column derivatization?
diastereomers resolve on HPLC and increase detection
require sample clean-up and pure derivatizing agent
what is other solution to separate enantiomer by HPLC?
chiral stationary phase
but expensive and not often used
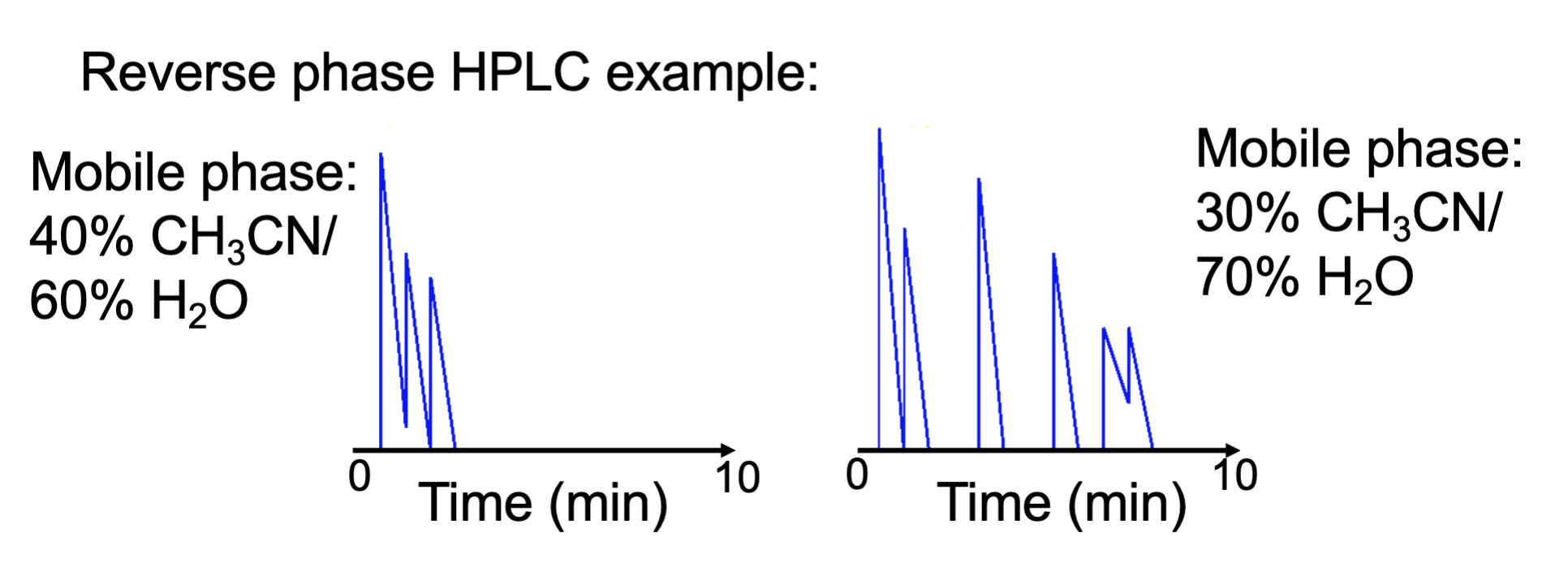
How do we optimize normal and reverse HPLC
change k—> a —> N order
change k is the easiest to manipulate
how? change mobile phase polarity to be less like analyte
gradient: can be continuous or step
What is the mobile and stationary phase of GC?
mobile: inert carrier gas (N2, He, CO2, Ar, H2)
stationary: starts as solid, melted into thin liquid layer coating column walls
What is the physiochemical requirement of sample used in GC
converted to gas phase
what are the physiochemical characteristics of GC? (hint: 2 characteristics)
terminally stable and chemically inert
base is usually siloxane based polymer
What happen to K and tr of nonpolar solutes when used hydrophobic stationary phase vs hydrophilic stationary phase in GC
larger K (more time in stationary phase) and longer tr for hydrophobic stationary phase and smaller K and shorter tr for hydrophilic stationary phase in GC
GC column temperature depends on
In GC, we focus on changing temperature as
analyte BP and degree of separation required
changing the mobile phase characteristics (carrier gas) are not possible
In GC, tr is dictated by
stationary liquid phase
column temperature
boiling point of the solute/analyte
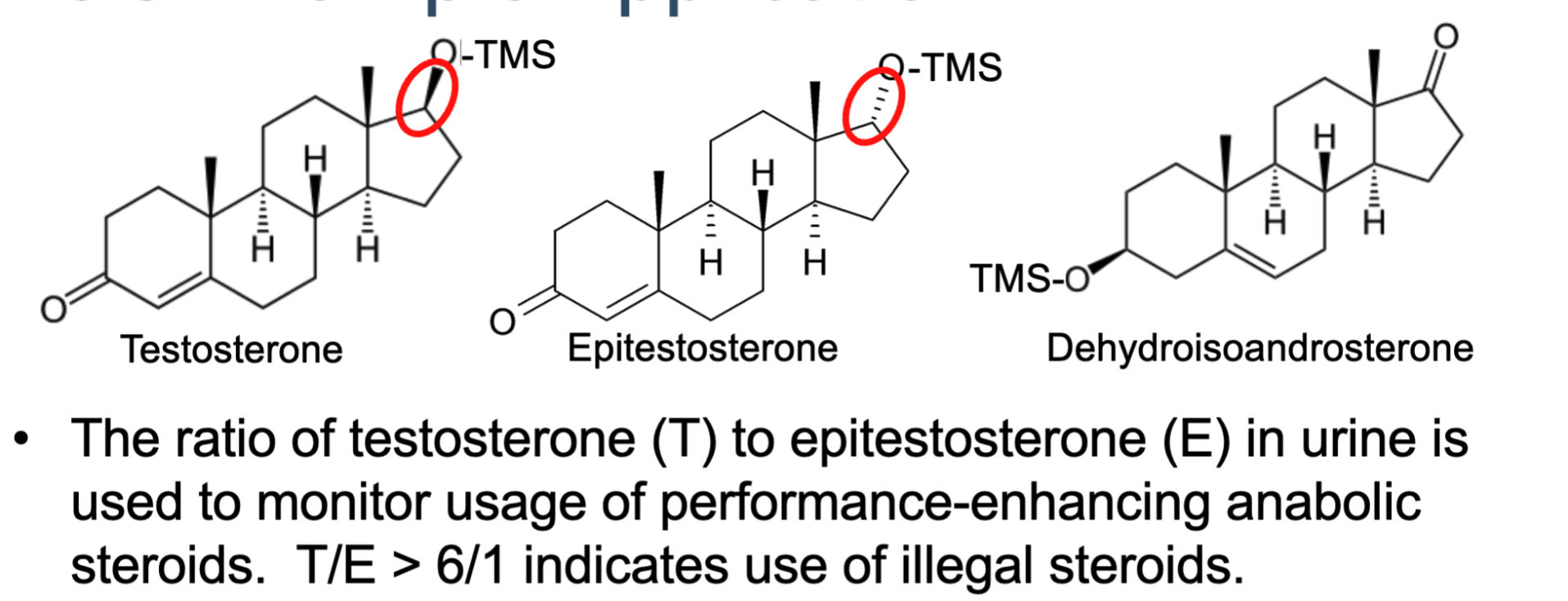
Explain the GC example in this image
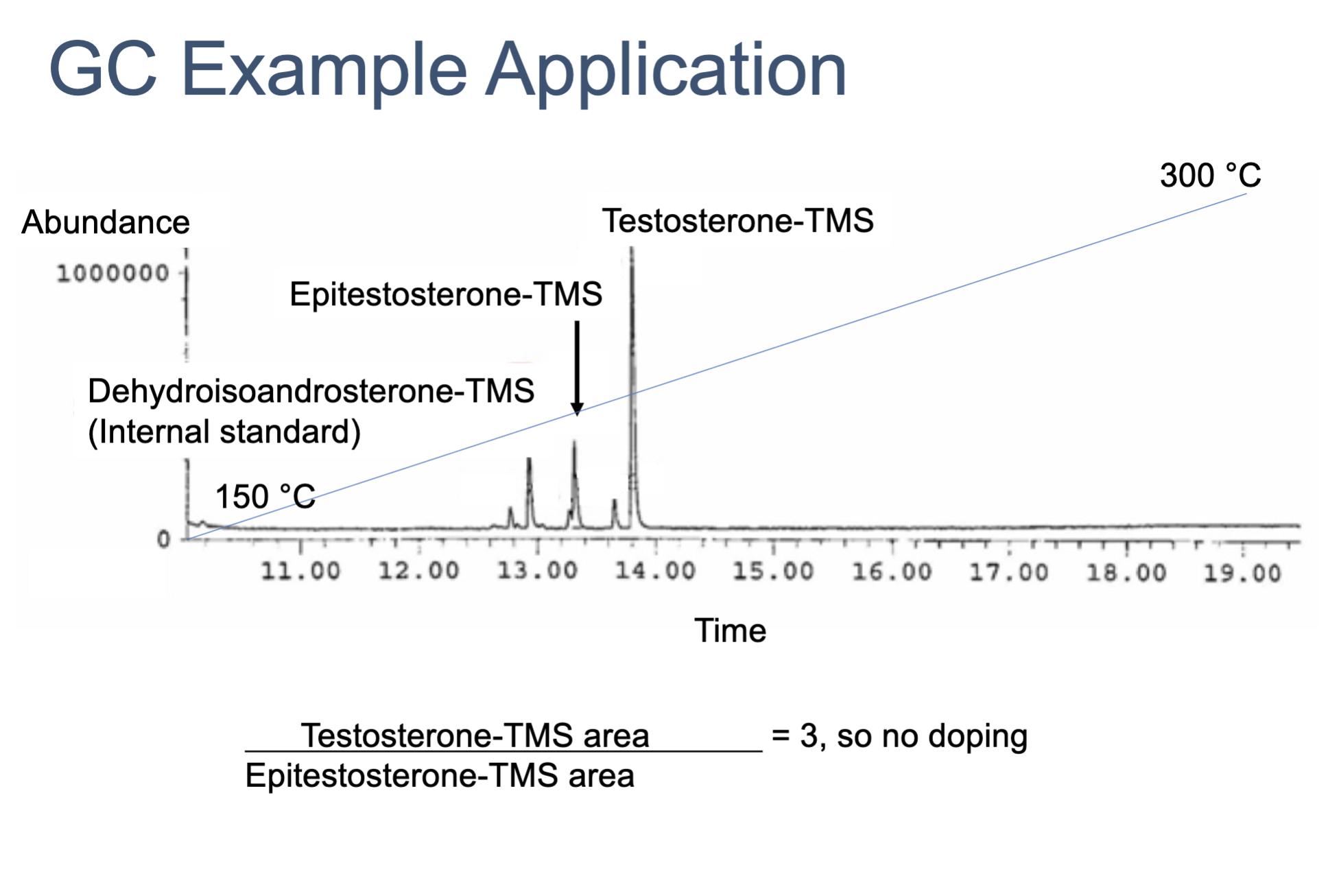

use the ratio to determine the quantity of epitestosterone and testosterone