3.1 - the photoelectric effect
1/75
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
76 Terms
what is the photoelectric effect?
when electromagnetic radiation above a certain frequency is directed at a metal, electrons are emitted from the metal surface as photoelectrons
what is directed at the metal in the photoelectric effect?
electromagnetic radiation
what is emitted from the metal surface during the photoelectric effect?
electrons, as photoelectrons
what surface is involved in the photoelectric effect?
metal surface
can the photoelectric effect occur at any frequency of the electromagnetic radiation?
no, the electromagnetic radiation must be of a certain threshold frequency
what is inside the metal?
delocalised (conduction) electrons, known as charge carriers
what are charge carriers?
the delocalised electrons inside the metal
what does the EM wave contain that causes the photoelectric effect?
lots of photons
which type of EM radiation is used for the photoelectric effect? why?
UV or x-ray, because they have the right frequency. UV is typically used
why are UV and x-rays used for the photoelectric effect?
because they have the right frequency
what is a photoelectron?
one photon reacting with one electron, that is then emitted from the metal surface
how many photons react with how many electrons?
ONE photon reacts with ONE electron
why is the photoelectron emitted?
because the photon has provided the electron with enough energy to escape the metal
what will happen if the photon doesn’t provide enough energy to exceed the work function?
the photoelectron will not escape. instead, the electron will collide with particles inside the metal until all it’s energy (as Ek) has dissipated. it does this because an electron can only pair with 1 photon at a time, some all the energy from the first photon must be dissipated before absorbing another
what was the problem with the discovery of the photoelectric effect?
because they could not be explained using the idea that light was a wave
what is threshold frequency (f0)?
the minimum frequency of incident EM wave for the photoelectric effect to occur
what does the threshold frequency depend on?
the type of metal
what is important to note about the wavelength of the incident EM light?
it must be less than the maximum value (that being c / f, where f is the threshold frequency) because λ = c/f
GET THIS EXPLAINED
what is the work function (Φ)?
minimum energy needed by an electron to escape the metal surface
what does the work function depend on?
the type of metal
what does the type of metal determine?
work function, Φ
threshold frequency, f0
what exactly is the energy of the work function?
the energy gained by the electron from the photon in the incident EM light, = hf
work function = ?
Φ = hf, because it is the energy gained by the electron from the photon in the incident EM light
what happens to the excess energy that exceeds the work function?
it becomes the photoelectron’s kinetic energy
kinetic energy of photoelectron
Ek max = hf - Φ
rules of the photoelectric effect
frequency of incident EM light must exceed threshold frequency
energy gained from photon must exceed work function
number of electrons emitted per sec is proportional to intensity (i.e., brightness, power emitted by light) of EM light (providing the first 2 rules are followed)
occurs immediately without decay, regardless of intensity (providing the first 2 rules are followed)
what is the number of electrons emitted per sec proportional to?
intensity (i.e., power emitted by the light, brightness) of the EM light (providing work function and threshold frequency are exceeded)
what is light intensity proportional to?
the number of electrons emitted per sec (providing work function and threshold frequency are exceeded)
how often does the photoelectric effect occur after incident EM light is shone?
immediately, without decay (providing work function and threshold frequency are exceeded)
how can we observe the photoelectric effect?
using a gold leaf electroscope
what does a gold leaf electroscope allow us to see?
the photoelectric effect
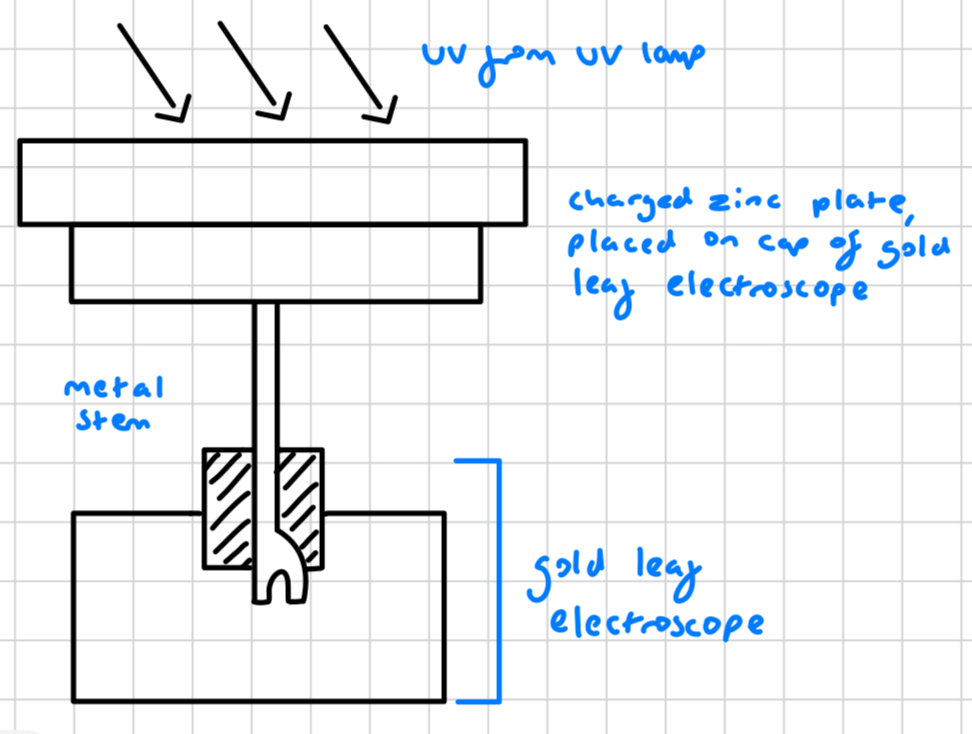
describe the gold leaf electroscope apparatus
a very sensitive detector of change
gold leaf electroscope with a metal stem
charged zinc plate placed on cap of gold leaf electroscope
UV radiation from UV lamp shone onto the charged zinc plate
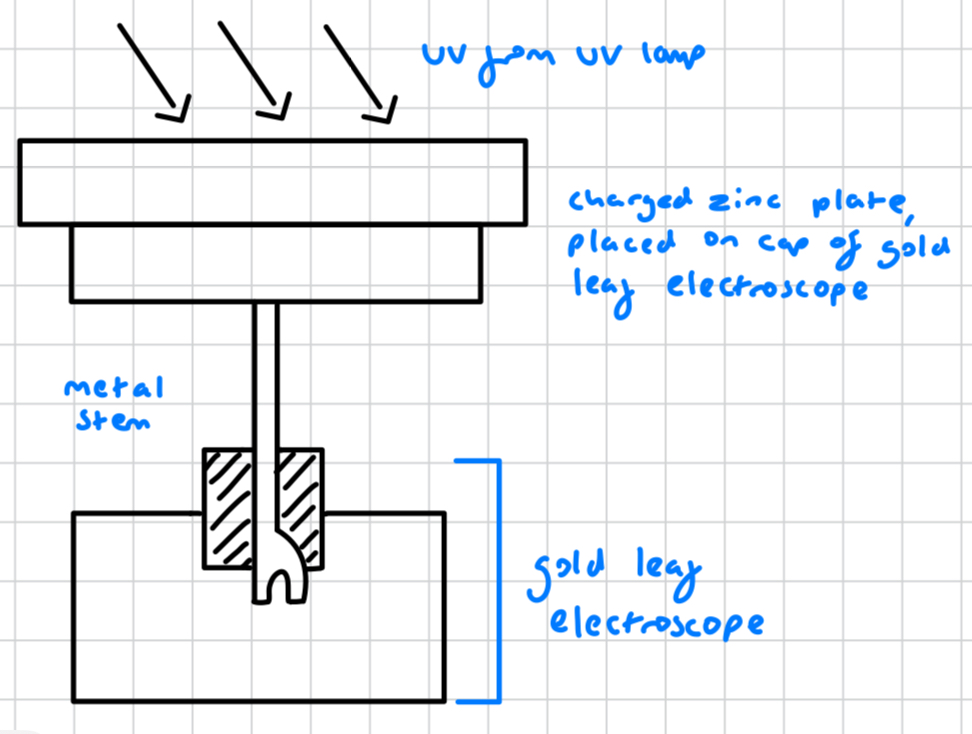
what is placed on the cap of the gold leaf electrolyte?
a charged zinc plate
what material is the charged plate on the cap of the gold leaf electrolyte?
zinc
is the zinc plate on the cap of the gold leaf electrolyte charged?
yes
what is the gold leaf electrolyte a detector of?
a very sensitive detector of change
is the gold leaf electrolyte a sensitive or insensitive detector of change?
very sensitive
what kind of radiation is used in the gold leaf electrolyte?
UV radiation from a UV lamp
where does the incident UV radiation come from in the gold leaf electrolyte experiment?
a UV lamp
where is the UV radiation shone on the gold leaf electrolyte apparatus?
the charged zinc plate
how does a gold leaf electrolyte work?
when the device is charged (either positively or negatively), both the thin gold leaf and metal stem have the same charge
the gold leaf and metal stem repel each other (cuz they have the same charge), raising the gold leaf and keeping it in that raised position
UV light can now be shone at the zinc plate
how does the charge of the thin gold leaf and the charge of the metal stem differ when the gold leaf electrolyte apparatus is charged?
it doesn’t - the thin gold leaf and the metal stem have the same charge
at what point is the charge of the thin gold leaf the same as the metal stem?
when the electrolyte apparatus is charged, either negatively or positively
why is the gold leaf thin in the gold leaf electrolyte apparatus?
ASK
what happens when the thin gold leaf and the metal stem have the same charge?
they repel each other, raising the gold leaf
why do the thin gold leaf and the metal stem repel each other?
because they have the same charge when the electrolyte apparatus is charged
what happens to the thin gold leaf when it repels with the metal stem?
it is raised and stays in this position
before the UV light is shone on the charged zinc plate, does the thin gold leaf stay raised depending on whether it is negatively or positively charged?
the gold leaf will remain raised both when it is negatively and positively charged
when UV light is shone on the charged zinc plate, what happens if the device is positively charged?
it will stay in the raised position regardless of the light
why does a positively charged gold leaf remain raised when a UV light in incident on it?
because it is negatively charged electrons that are emitted in the photoelectric effect, therefore photoelectron effect doesn’t occur and the gold leaf stays raised
when UV light is shone on the charged zinc plate, what happens if the device is negatively charged?
the gold leaf will gradually fall from it’s raised position
why does a negatively charged gold leaf gradually fall from it’s raised position when a UV light in incident on it?
photoelectric effect occurs when light is incident, as photons from UV pair with the electrons in the negatively charged zinc plate
electrons in zinc emitted as photoelectrons
zinc’s magnitude of charge decreases with the more emitted photoelectrons
so the gold leaf is gradually less repulsed by the negatively charged metal, the repulsive force decreases, and the gold leaf gradually falls down
when UV light is incident, why does the thin gold leaf fall gradually when the zinc plate is negatively charged?
the speed of the gold leaf’s decent is proportional to the amount of photoelectrons emitted, so the thin gold leaf falls further down in pace with the total number of photoelectrons emitted
CHECK THIS IS RIGHT
what is stopping potential (vs)?
the minimum potential (energy) needed to stop photoelectron emission
what can happen if the zinc plate has a sufficient positive charge?
the plate can attract the negatively charged photoelectrons back to the metal
why does the zinc plate need a sufficient positive charge to stop the photoelectric effect (stopping potential)?
because the photoelectrons are negatively charged, so a sufficient positive charge from the plate will attract the negatively charged photoelectrons back to the metal surface
what happens to the energy of the photoelectron when the metal plate is at / exceeds the stopping potential?
the photoelectron’s Ek is reduced to 0
why is the photoelectron’s Ek reduced to 0 when the plate is at the stopping potential?
because each emitted photoelectron does work = e x vs to leave the metal surface (as it must overcome the stopping potential to be emitted), hence it’s Ekmax = e x vs
GET THIS EXPLAINED
what does a photoelectron have to overcome to be emitted?
it’s energy must overcome the stopping potential
what is the work done by each emitted electron = to?
work done by each emitted electron = e x vs
GET THIS EXPLAINED. what does e = ?
what is the Ekmax of an emitted photoelectron?
Ekmax = (e) x stopping potential (vs)
WHAT DOES E MEAN
photoelectric effect equations
λ of incident light < maximum value (= c/f)
c = speed of light
f = frequency of threshold frequency, f0
energy of photon (E) = hf = (hc) / λ
h = planck’s constant, 6.63 × 10-34 Js
f = frequency of incident light
c = speed of EM waves
λ = wavelength of incident light
Ekmax of emitted electron = hf - Φ
h = planck’s constant, 6.63 × 10-34 Js
f = frequency of EM waves DOUBLE CHECK
Φ = work function
threshold freq, fmin / 0 = Φ / h
Φ = work function
h = planck’s constant, 6.63 × 10-34 Js
λ of incident light > ?
energy of photon (E) = hf = (hc) / λ
h = planck’s constant, 6.63 × 10-34 Js
f = frequency of incident light
c = speed of EM waves
for the maximum wavelength of incident light, λ = c/f, what do the symbols represent?
λ = wavelength of incident light
c = speed of light
f = threshold frequency
energy of photon (E) = ?
energy of photon (E) = hf = (hc) / λ
h = planck’s constant, 6.63 × 10-34 Js
f = frequency of incident light
c = speed of EM waves
for the energy of a photon, E = hf = (hc) / f, what do the symbols represent?
energy of photon (E) = hf = (hc) / λ
h = planck’s constant, 6.63 × 10-34 Js
f = frequency of incident light
c = speed of EM waves
λ = wavelength of incident light
Ekmax of emitted electron = ?
Ekmax of emitted electron = hf - Φ
h = planck’s constant, 6.63 × 10-34 Js
f = frequency of EM waves DOUBLE CHECK
Φ = work function
for the Ekmax of an emitted electron, Ekmax = hf - Φ, what do the symbols represent?
h = planck’s constant, 6.63 × 10-34 Js
f = frequency of EM waves DOUBLE CHECK
Φ = work function
fmin = ?
threshold freq, fmin / 0 = Φ / h
Φ = work function
h = planck’s constant, 6.63 × 10-34 Js
for the threshold frequency, fmin = Φ / h, what do the symbols represent?
Φ = work function
h = planck’s constant, 6.63 × 10-34 Js
why does fmin = Φ / h ?

Ekmax of emitted photoelectron > 0
Ekmax of emitted photoelectron ≥ 0
which is it? why?
Ekmax of emitted photoelectron > 0, because the photoelectron must have some Ek to move out of the metal surface during emission
why must an emitted photoelectron have at least some Ek ?
to move out of the metal surface during emission
what is the photoelectric effect proof of?
the light-particle theory
why does the photoelectric effect prove against the light-wave theory?
if light were a wave, then each electron would gain some energy from incoming waves regardless of the light’s frequency, except they don’t and instead there is a threshold frequency
why does the photoelectric effect prove the light-particle theory?
the existence of a threshold frequency - as if light were a wave, then each electron would gain some energy from incoming waves regardless of the light’s frequency, except they don’t and instead there is a threshold frequency
the photoelectric effect occurs without delay - as if light were a wave, then GET THIS EXPLAINED