CHEMISTRY UNIT 1 Structure and Bonding
1/34
Earn XP
Description and Tags
Natures Chemistry - Structure and Bonding
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
35 Terms
What are intramolecular bonds ?
Intramolecular bonds are the type of bonds inside molecules
What are intermolecular bonds ?
Intermolecular bonds are the types of bonds between molecules
What is a covalent bond and how does it form ?
A covalent bond is a shared pair of electrons, electrostatically attracted to the positive nuclei of two atoms.The covalent bond is a result of two positive nuclei being held together by their common attraction for the shared pair of electrons
What are the types of intramolecular bonding ?
pure covalent bonds
polar covalent bonds
ionic bonds
What are polar covalent bonds and why do they form ?
A polar covalent bond is the type of bond formed when a shared pair of electrons are not shared equally. This is due to one of the elements having a higher electronegativity than the other.
What are pure covalent bonds ?
Pure covalent bonds are bonds that exist between two atoms with the same electronegativities.
What is a pure covalent bonds ionic character ?
A pure covalent bond has no ionic character at all
What are examples of pure covalent bonds ?
diatomic elements ( Hydrogen, Oxygen, Fluorine ect)

What type of bond is this and why?
Polar covalent bond. This is because the shared pair of electrons between an atom of hydrogen and an atom of bromine are not shared equally. Since bromine has a greater electronegativity than hydrogen, it will pull the bonding electrons towards itself.
What is a dipole?
A dipole is a molecule with its opposite ends having different charges ( one with a delta + charge and the other with a delta - charge)
What are ionic bonds ?
Ionic bonds are the electrostatic attraction between positive and negative ions.
What do ionic compounds form ?
Ionic compounds form three-dimensional ionic lattice structures of positively and negatively charged ions
What are ionic bonds formed between ?
Ionic bonds are usually (but not always) formed between a metal and a non-metal with a large difference in electronegativity. (eg Sodium Chloride)
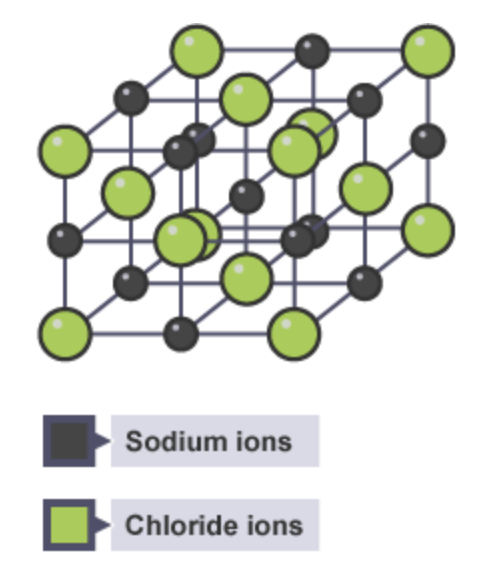
What type of bond is this and why ?
This is an Ionic bond. This is because Chlorine has a far larger electronegativity and so it pulls the bonding electrons towards itself completely, thus gaining an electron and forming a negative ion. Sodium, due to its low electronegativity, will lose its bonding electron to chlorine and form a positive ion.
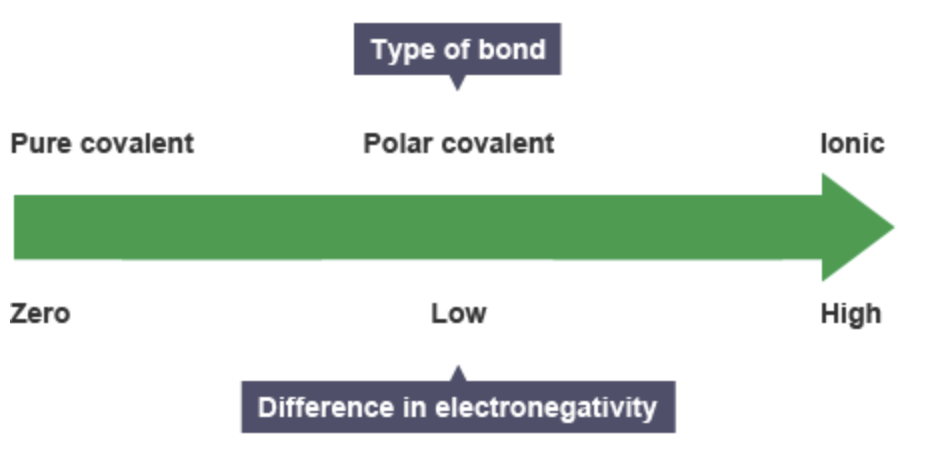
What is this and what is it used for
The bonding continuum is used to determine the type of bonding present in a compound by the differences in electronegativity between the elements involved. Pure covalent bonds, polar covalent bonds and ionic bonds all exists as part of the same bonding continuum.
What are intermolecular bonds ?
Intermolecular bonds are found between the molecules
What are the types of intermolecular bonds?
London dispersion forces (LDFs)
Permanent dipole-permanent dipole interactions (PD-PD interactions)
Hydrogen bonding
What are London Dispersion Forces and how do they exist?
London dispersion forces are forces of attraction that can operate between all atoms and molecules and they are formed as a result of electrostatic attraction between temporary dipoles and induced dipoles caused by movement of electrons in atoms and molecules
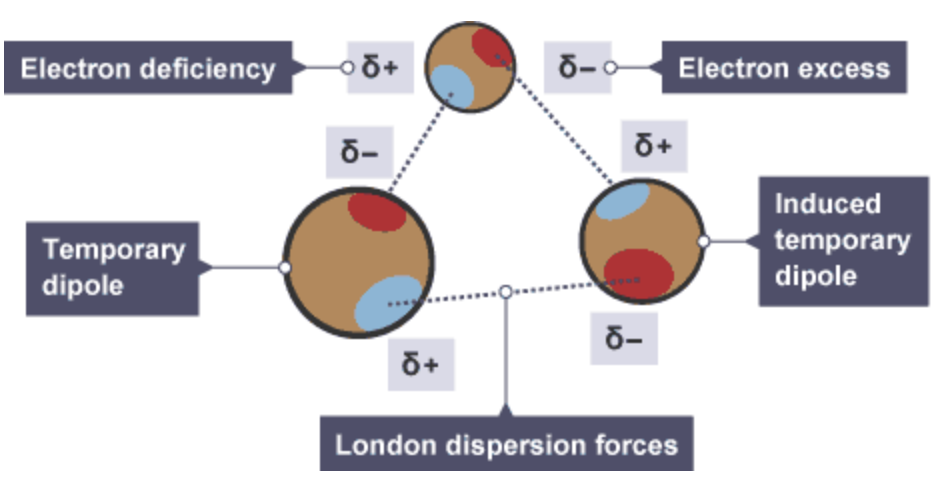
Are London Dispersion Forces weak or strong ?
London Dispersion Forces are the weakest type of intermolecular bond
What does the strength of London Dispersion Forces relate to ?
The strength of London dispersion forces is related to the number of electrons within an atom or molecule
What are molecules that have a permanent dipole called?
Molecules with a permanent dipole are polar
How strong are permanent dipole-permanent dipole interactions ?
Permanent dipole-permanent dipole interactions are stronger than LDFs with the same number of electrons
What are permanent dipole-permanent dipole interactions ?
Permanent dipole-permanent dipole interactions are electrostatic attractions between polar molecules, where the slightly positive (δ+) end of one molecule is attracted to the slightly negative (δ−) end of a neighbouring molecule.
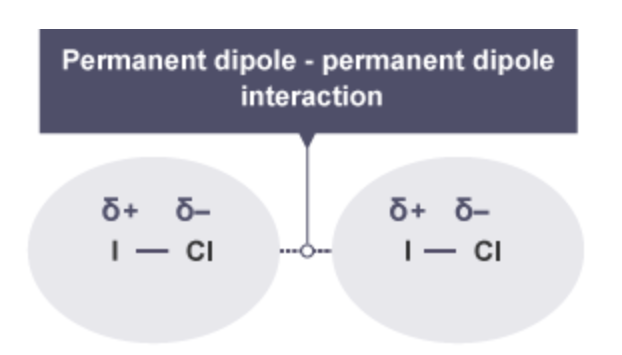
What do polar molecules display ?
Polar molecules display attractions between the oppositely charged ends of the molecules
What is hydrogen bonding ?
It is a specific type of permanent dipole to permanent dipole attraction that occurs when a hydrogen atom is covalently bonded to a highly electronegative element such as nitrogen, oxygen or fluorine. (NOF) and the same as permanent dipole-permanent dipole interactions, the oppositely charged ends of molecules attract.
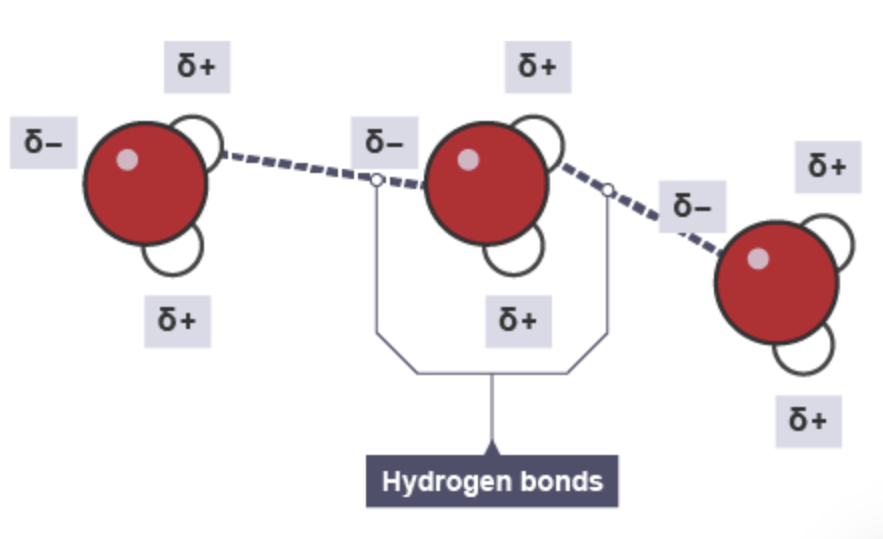
How strong is hydrogen bonding ?
A hydrogen bond is stronger than other forms of permanent dipole-permanent dipole interaction but weaker than a covalent bond
What are examples of compounds which contain hydrogen bonding ?
water
ammonia
alcohols
alkanoic acids
What are the relative strengths of bonds ?
Covalent bonds
|
Hydrogen bonds
|
Permanent dipole-permanent dipole interactions
|
London Dispersion Forces
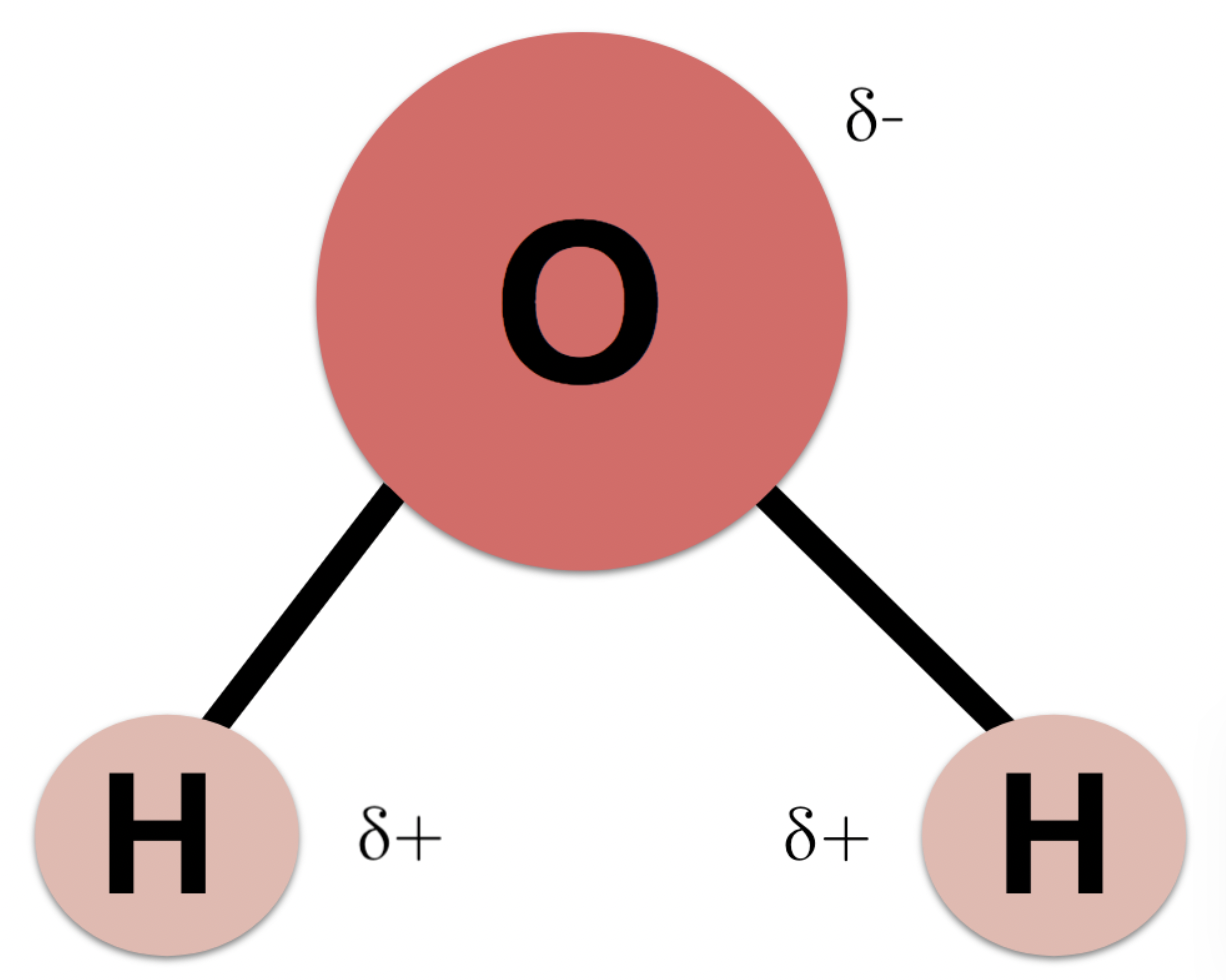
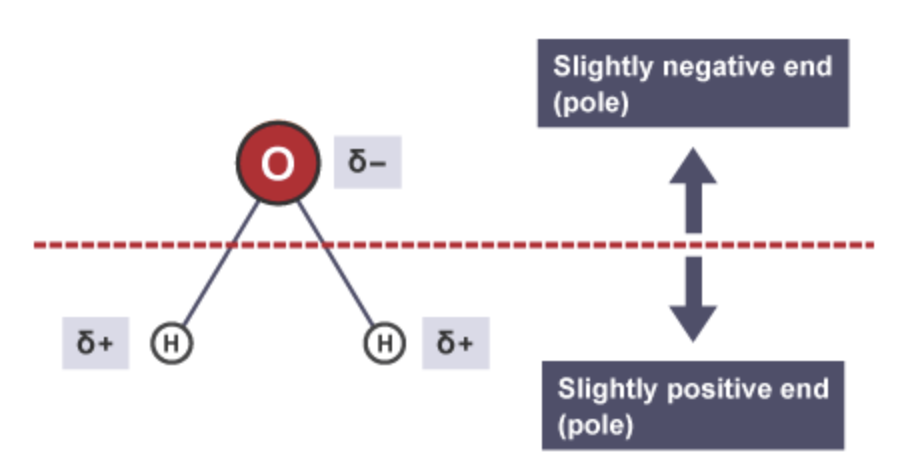
Are water molecules polar ?
Water molecules are polar molecules as both of the bonds inside the molecule are polar bonds and due to the non-symmetrical shape of the molecule (bent) the molecule itself is polar (it has a delta - and delta + side)
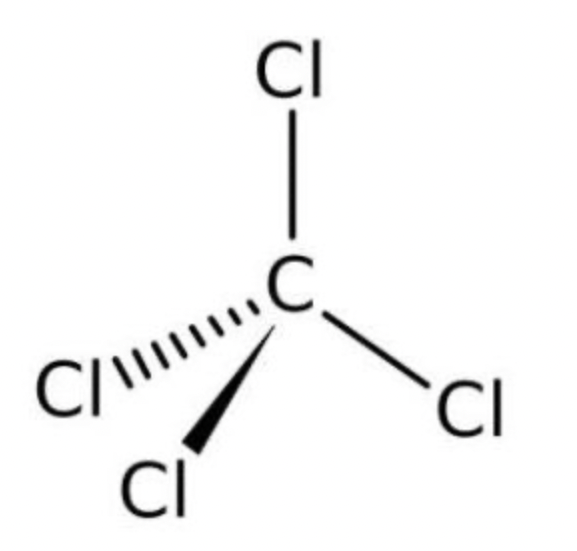
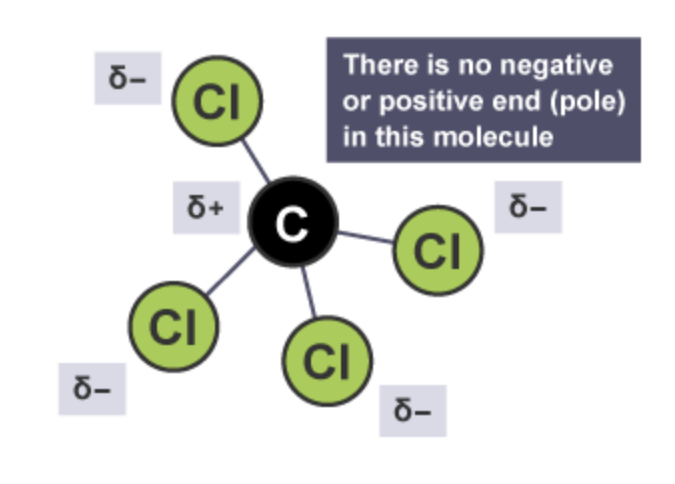
Is the carbon tetrachloride molecule polar ?
Carbon tetrachloride contains four polar covalent bonds, however because it is a tetrahedral molecule and the charges are symmetrical the delta + part of the molecule (carbon atom) is locked away at the centre of the molecule and no matter what direction you approach the molecule there is no negative or positive end (pole) in this molecule. This means that the molecule itself is non-polar due to symmetry)
Properties relating to ionic lattice structures (3)
high melting & boiling point
conduct when molten or in solution as ions are free to move
can be broken down by electrolysis
Properties relating to Covalent Network (2)
very high melting & boiling points
do not conduct electricity
Properties relating to covalent molecular
low melting & boiling points
do not conduct electricity
Why do ionic lattices conduct electricity when molten or in solution ?
Ionic lattices conduct electricity when molten or in solution because the ions are free to move
Why can covalent molecular molecules that seem very similar can have very different properties ?
Covalent molecular molecules that seem very similar can have very different properties because of the intermolecular bonds present