UnitA - Intermolecular forces, spectrum, solubility
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
16 Terms
Define intermolecular bonds
Forces between molecules
What properties are intermolecular forces responsible for?
Physical properties and behaviour such as: melting/boiling point and solubility of substances
What is the difference between intra and inter molecular bonds?
Intra - bonds between atoms within a molecule
Inter - bonds between molecules
What are the 3 types of intermolecular forces? What are their electronegativities and polarity?
London Dispersion Forces(weakest force)
lowest electronegativity difference
non-polar
Dipole-dipole Forces(medium force)
medium electronegativity difference
polar
Hydrogen Bonding(strongest force)
highest electronegativity difference
very polar
What kind of compounds have London forces?
All molecules
What determines a greater london dispersion force?
The larger the compound = more protons and electrons it has = greater the LDF = great boiling point
How do you know if the compound is dipole-dipole?
If the compound is polar, if it is non-polar, than no
What compounds have dipole-dipole forces?
Polar molecules that are electrostatically attracted to one another
The greater the difference in electronegativities = the stronger the dipole
How do you know if the compound is hydrogen bonding?
If hydrogen is directly attached to fluorine, oxygen, or nitrogen
What compounds have hydrogen bonding?
H-F, H-O, H-N
How do you know if the compound has a high boiling point?
the bigger the compound = more protons and electrons = bigger the LDF = big boiling points
When determining which molecule has the biggest boiling point, but they are the same size, you should…
Look at how many different intermolecular forces it has
If the compound 3 types of forces, it has the higher boiling point
How do e- act in non-polar bonds?
e- are held by equally sharing e- between atoms
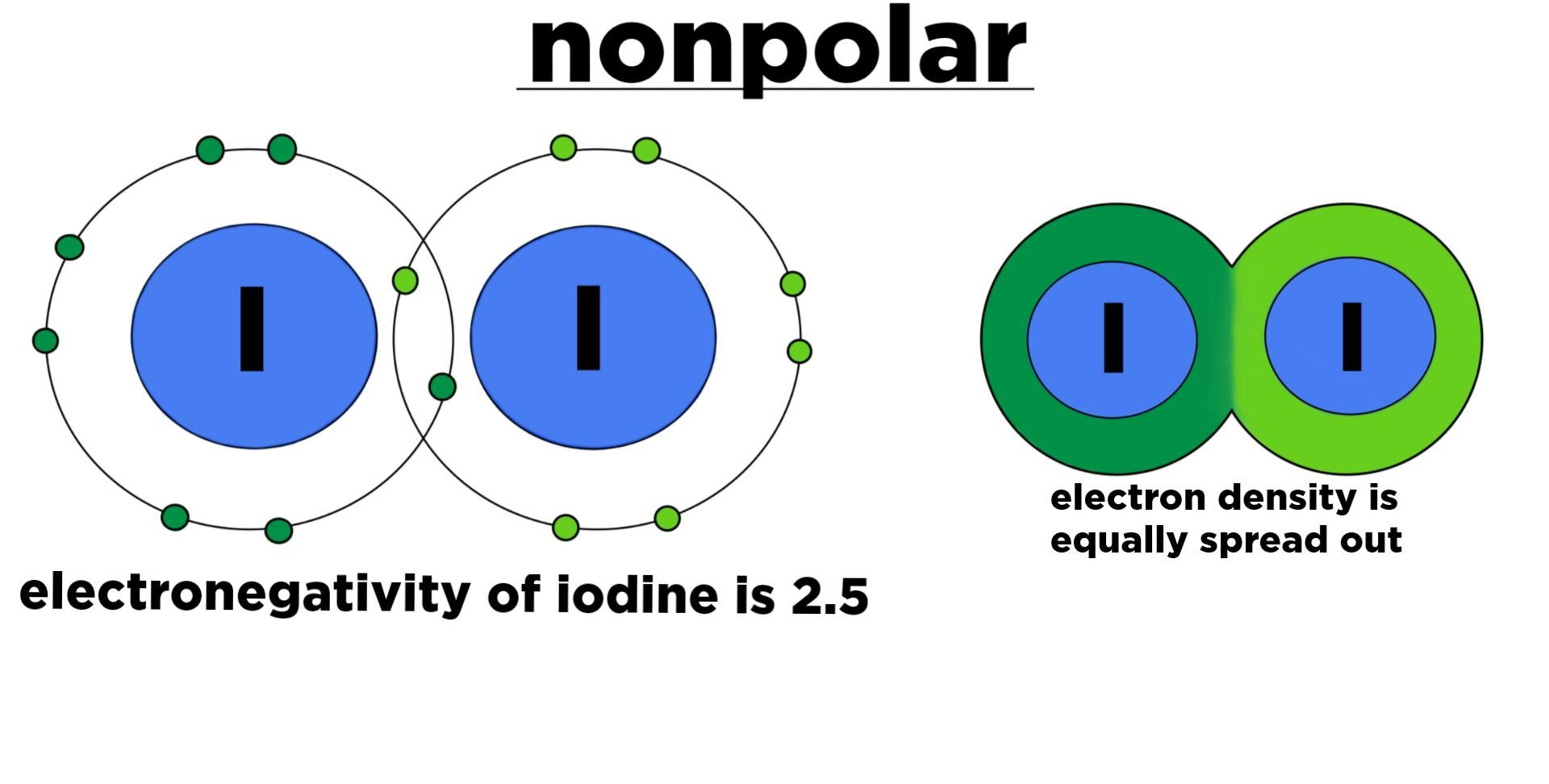
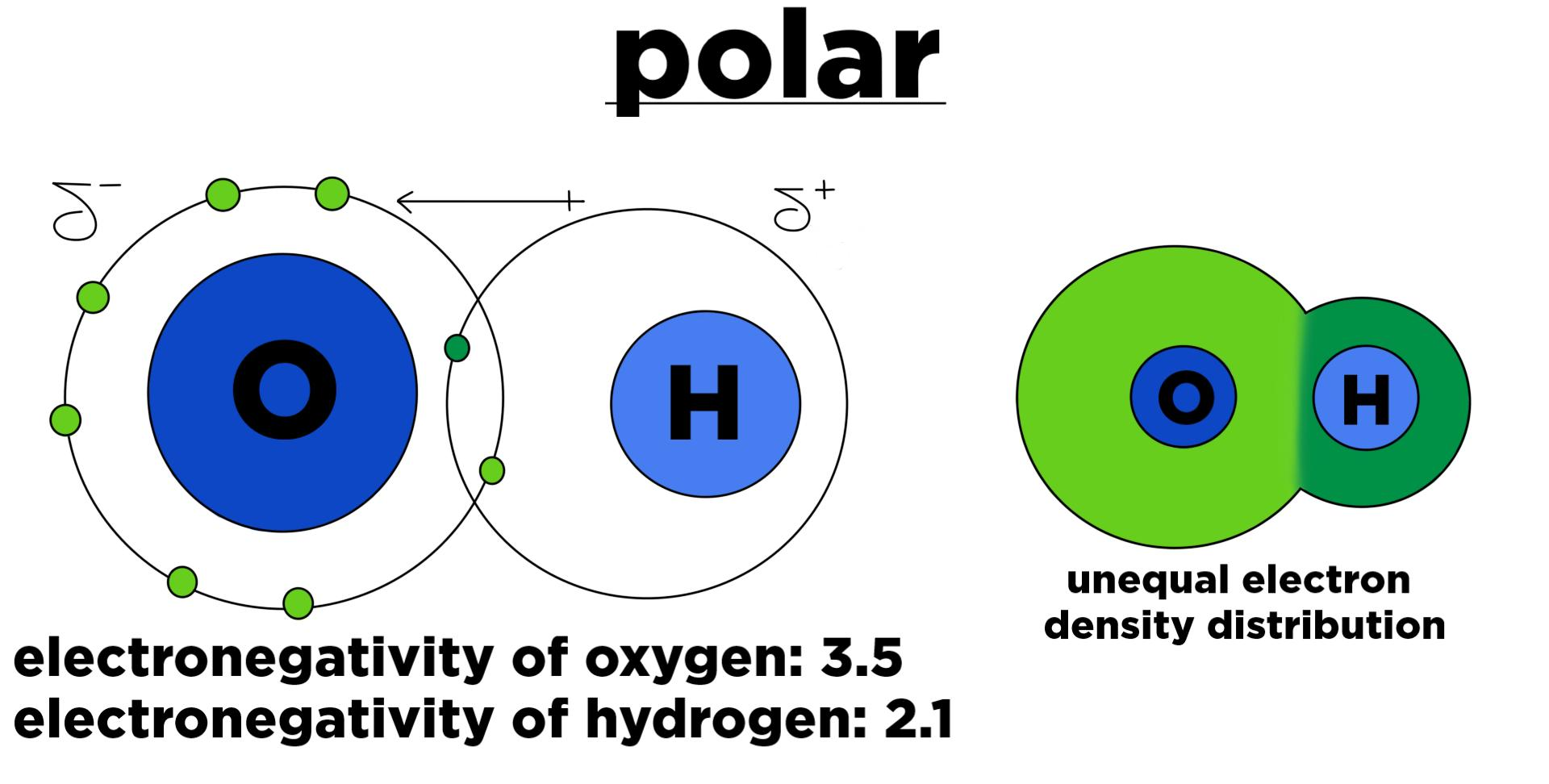
How do e- act in polar bonds?
e- are shared unequally where e- are slightly towards one side
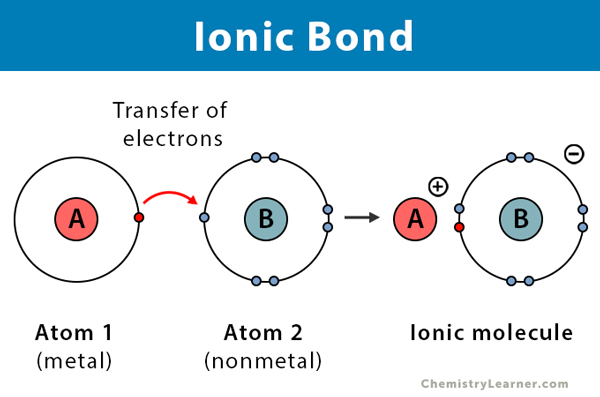
How do e- act in ionic bonds?
e- are dragged and transferred towards one side
What solvent are polar and non-polar solutes able to dissolve in?
Like dissolves like
Polar things dissolve in polar compounds
Non-polar things dissolve in non-polar compounds