Biology - Biological Molecules (1)
1/199
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
200 Terms
Atomic mass - total number or protons and neutrons in an atom

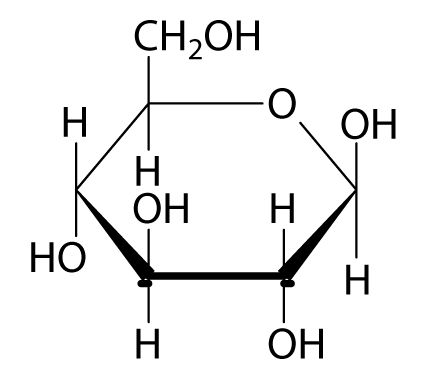
What are the 3 steps for reducing sugars carried out?
1 - Add 2cm^3 of food sample to test tube 2 - Add equal volume Benedict's reagent 3 - Heat mixture in a gently boiling water bath at 80C for 5 minutes
What are the 7 steps to detecting a non-reducing sugar?
1 - Grind sample up and add water so in solution 2 - Add 2cm^3 of food sample to 2cm^3 of Benedict's reagent in a test tube and filter 3 - Place test tube in water bath (80C) for 5 minutes, if solution doesn't change colour/remains blue, then a reducing sugar is NOT present 4 - Add another 2cm^3 of food sample to 2cm^3 of dilute HCl in a test tube and place in water bath (80C) for 5 minutes, the HCl will hydrolyse any disaccharide into monosaccharide components 5 - Slowly add some sodium hydrogencarbonate to test tube to neutralise the HCl (Benedict's reagent won't work in acidic conditions), test with pH paper to ensure solution is alkaline 6 - Re-test the solution by heating it with 2cm^3 of Benedict's reagent in a water bath (80C) for 5 minutes 7 - If a non-reducing sugar was present in original sample, the Benedict's reagent will turn orange-brown, due to the reducing sugars produced from hydrolysis of non-reducing sugars
2 - Add 2 drops of iodine solution and shake/stir
3 - The presence of starch is indicated by a blue-black coloration
Pentose - 5C e.g. ribose, deoxyribose
Hexose - 6C e.g. glucose, fructose
- A glycosidic bond forms between the two monosaccharides and water is released
- This energy can be used to produce ATP
- The breaking of glucose is carried out in a few small steps , each step requires an enzyme
- Animals and plants contain enzymes which can break alpha-glucose but not beta-glucose
- Molecules contain 300 glucose molecules and adopt a helical shape, this is due to the shape of the glucose molecules and formation of glycosidic bonds
- Forms a compact shape and is stored in a small space
- Large molecule and insoluble in water (will not diffuse out of cell and doesn't affect water potential/osmosis)
- Branches occur due to formation of 1,6 glycosidic bonds every 20-30 monomers, creates a branched and coiled into a compact shape
2 - It is insoluble, it does not diffuse out of cells
3 - It is insoluble, it prevents high concentration of glucose in cells = doesn't affect water potential of the cell
4 - Easily hydrolysed into simple glucose monomers, due to branched ends which can be respired and energy released
- Macrofibrils allow water to move through and along cell walls (movement of water - apoplast pathway)
- Prevent lysis (bursting) of the cell
- Arrangement of macrofibrils in guard cells allows opening and closing of stomata
- Cell walls can be reinforced with other substances e.g. lignin
- A few organisms possess cellulase e.g. fungi
- Ruminant animals depend upon large populations of bacteria to break down cellulose
- Peptidoglycan/murein - forms cell walls in bacterial cells