Neuroscience of Drug Addiction - Exam 2
1/348
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
349 Terms
Intracranial Self Stimulation (ICSS)
A behavioral paradigm where animals can administer electrical stimulation to specific brain regions, often associated with the brain's reward pathways, demonstrating the reinforcing effects of stimulation.
highlights the role of certain areas of the brain in motivation and reward-seeking behavior, frequently studied in the context of drug addiction.
Brain Stimulation Reward (BSR)
Electrical self-stimulation designed to provide a reward that reinforces the action of pressing a level
the intended effect/outcome of ICSS
Common ICCS sites:
Basal forebrain
Nucleus accumbens
Medial forebrain bundle
Ventral midbrain
Dorsal brainstem
Locus Coeruleus (LC)
A nucleus in the brainstem involved in stress responses and the regulation of arousal, attention, and the release of norepinephrine, which plays a role in addiction and reward pathways
Substantia Nigra (Sn)
A dopamine-rich area in the midbrain that plays a crucial role in the regulation of movement and reward, often associated with addiction and Parkinson's disease.
Ventral Tegmental Area (VTA)
A group of neurons located in the midbrain that plays a key role in the reward circuit, releasing dopamine and influencing motivation, pleasure, and addiction.
Ventral Midbrain
The region of the midbrain that contains important structures such as the substantia nigra and ventral tegmental area, involved in the regulation of movement, reward, and addiction.
Medial Forebrain Bundle (MFB)
A major neural pathway in the brain that connects the ventral tegmental area to other regions, namely the NAc, playing a significant role in the reward system and the effects of addictive substances.
Nucleus Accumbens (NAc)
A key structure in the brain's reward circuit, the nucleus accumbens is involved in processing rewards, reinforcing pleasurable experiences, and is heavily influenced by dopamine
plays a crucial role in addiction and motivation.
Basal Forebrain
A collection of structures in the forebrain involved in the regulation of arousal, attention, and the processing of rewards, playing a significant role in learning and memory.
Dorsal Brainstem
A region in the brain responsible for regulating basic life functions such as breathing, heart rate, and arousal; it connects higher brain functions with spinal cord activities.
Septum
A region in the brain associated with the limbic system, the septum is involved in emotional regulation and has a role in mediated rewarding and pleasurable behaviors.
Stimulation Frequency
The rate at which electrical impulses are delivered to neurons, influencing the intensity of responses and the potential for synaptic plasticity in the context of addiction and learning.
titrated systematically to identify the minimum frequency that reinforces instrumental responding (reward threshold)
Reward Threshold
The minimum level of stimulation required to elicit a reward response, often influencing behavior in the context of addiction and reinforcement.
serves as an index of the hedonic state
How does acute exposure to drugs of abuse (DOA) affect the reward threshold?
They can lower the reward threshold, making individuals more sensitive to reinforcement and increasing the likelihood of engaging in drug-seeking behaviors.
Hedonic State
A state of pleasure or satisfaction that influences motivation and behavior, particularly in the context of addiction.
Voluntary Drug Taking
The behavior of individuals choosing to consume drugs despite negative consequences, often linked to addiction and reinforcement mechanisms.
Passive Drug Exposure
Exposure to drugs without active use, leading to potential behavioral or physiological effects.
Why might passive drug studies be more useful than those that capture voluntary drug taking?
Passive drug studies can provide insights into the effects of drugs in non-consumptive contexts, revealing potential health risks and societal impacts without the confounding factors of voluntary consumption.
they help understand underlying mechanisms of addiction and behavioral changes in environments with drug availability.
Instrumental Drug Self-Administration
A method of drug consumption where individuals actively administer substances to achieve specific effects or goals, often used in research to study addiction.
Operant conditioning model
Schedules of Reinforcement
Rules determining how and when reinforcements are given, influencing behaviors.
Fixed Ratio Reinforcement
A schedule where reinforcement is provided after a specific number (n) of responses, promoting a high rate of responding.
For example, providing a reward after every fifth behavior.
Variable Ratio Reinforcement
A schedule where reinforcement is provided after an unpredictable number of responses, leading to high and steady rates of responding.
This schedule is commonly observed in gambling scenarios
Generally varies around some mean
Fixed Interval Reinforcement
A schedule where reinforcement is given after a fixed amount of time has passed, encouraging responses as the time for reinforcement approaches.
This schedule often results in a pause after reinforcement followed by accelerating responses.
Variable Interval Reinforcement
A schedule where reinforcement is provided after an unpredictable amount of time has passed, which leads to moderate and steady rates of responding.
This schedule is often observed in situations like checking for a text message.
Cumulative Response Plots
Graphs that display the total number of responses over time, showing the rate of responding and the effects of reinforcement schedules.
What kind of reinforcement response do fixed ratio’s produce?
Typically produces a high rate of responding, with short pauses after each reinforcement in a linear trajectory.
This schedule encourages quick repetitions of the desired behavior to receive rewards.
What kind of reinforcement response do fixed interval’s produce?
Typically produces a moderate, steady rate of responding, with long pauses after reinforcement.
Often described as ‘scalloped
This schedule encourages consistent behavior over time to receive rewards.
Progressive Ratio (PR)
A reinforcement schedule in which the requirement for reinforcement increases progressively, often leading to behavior extinction as individuals may stop responding once the effort outweighs the reward
Breakpoint
The point at which an individual stops responding in a progressive ratio schedule due to increasing effort for reinforcement (i.e. giving up)
What are the advantages of a progressive ratio schedule?
Easier to interpret than a fixed ratio
Can readily compare different drugs
What are the limitations of a progressive ratio schedule?
Just one data point for a given dose
Complex cumulative dosing
Uncontrolled Drug Use
Continued consumption of drugs despite negative consequences, often leading to addiction and health issues.
Compulsive Drug Use
A pattern of using drugs that is characterized by an inability to control consumption despite negative consequences.
It often leads to habitual and unrelenting pursuit of drug-seeking behavior.
Example of a model of excessive drug intake:
Model in which a ‘short access’ (ShA) group & a ‘long access’ (LgA) group were compared
the ShA group was only allowed to self-administer for 1 hour/day
the LgA group was allowed to self-administer for 6 hours/day
Escalation Effect
The phenomenon where an individual progressively increases their drug intake over time, often leading to higher doses to achieve the desired effect.
Intermittency
A characteristic of drug intake that may result in increased cravings and heightened sensitivity to drug stimuli following long periods between doses
Intermittent Access
A schedule of drug administration that alternates between periods of access and abstinence, which can enhance drug-seeking behavior and cravings.
How does intermittent access to self-administration affect addiction-like behavior?
It increases addiction-like behavior by inducing a heightened state of craving and reinforcing the desire for the drug, as individuals experience periods of deprivation followed by drug access.
Models of Drug Craving & Relapse
Theoretical frameworks that explain how the desire for drugs can emerge and lead to relapse, highlighting the roles of environmental cues, emotional states, and individual differences in addiction vulnerability.
Cue
A stimulus or environmental context associated with drug use that can trigger cravings or relapse in individuals recovering from addiction.
Stress
A physical or psychological response to perceived challenges or threats that can exacerbate drug craving and increase the risk of relapse in individuals with addiction.
Drug Priming
A phenomenon where exposure to a small dose of a drug can trigger cravings and reinstatement of drug-seeking behavior in individuals with a history of addiction, regardless of their current abstinence.
Extinction
The process in which conditioned responses, such as drug cravings, diminish or disappear when the associated cues are repeatedly presented without the drug.
Reinstatement Model
A research model used to study the relapse of addictive behaviors, where drug-seeking behavior is triggered after a period of abstinence through exposure to drug-related cues or stressors.
Classical Neurotransmitters
Amino Acid Neurotransmitters
Monoamines
Acetylcholine (ACh)
Purines
Nonclassical Neurotransmitters
Neuropeptides
Lipids
Gases
Amino Acid Neurotransmitters
The primary neurotransmitters in the central nervous system, consisting of:
glutamate
gamma-aminobutyric acid (GABA)
glycine
Monoamines
A group of neurotransmitters that play crucial roles in the brain's reward system and emotional processing, including
dopamine (DA)
norepinephrine (NE)
serotonin (5-HT)
histamine (HA)
Acetylcholine (ACh)
A neurotransmitter involved in muscle contraction, cognition, and memory, functioning in both the central and peripheral nervous systems
Purines
A class of neurotransmitters that play a role in energy transfer and modulation of neuronal activity, including:
adenosine
ATP
Neuropeptides
A diverse group of signaling molecules in the nervous system that modulate a variety of physiological processes, including pain, reward, and stress responses, includes:
endorphins & enkephalins
corticotropin-releasing factor (CRF/CRH)
orexin
brain-derived neurotrophic factor (BDNF)
vasopressin
oxytocin
many more
Lipid Neurotransmitters
A group of signaling molecules derived from lipids that play important roles in cellular communication and modulation of neurotransmission, including
anandamide
2-AG
Gaseous Neurotransmitters
A class of signaling molecules that diffuse freely across cell membranes, playing roles in neurotransmission and modulation of neuronal activity, including
nitric oxide (NO)
carbon monoxide (CO)
hydrogen sulfide (H2S)
Glutamate
The most abundant excitatory neurotransmitter in the brain, playing a crucial role in synaptic plasticity, learning, and memory
GABA
The primary inhibitory neurotransmitter in the brain, critical for reducing neuronal excitability and regulating muscle tone
Glycine
An inhibitory neurotransmitter found in the central nervous system, playing a role in reducing neuronal excitability and regulating motor and sensory functions
Dopamine (DA)
A key neurotransmitter involved in reward, motivation, and the regulation of mood
is crucial in pathways associated with pleasure and addiction, often linked to the effects of drugs.
Norepinephrine (NE)
A neurotransmitter that plays a significant role in the body’s response to stress, influencing alertness, arousal, and emotional regulation
involved in attention, focus, and the body's fight-or-flight response.
Serotonin (5-HT) as a Neurotransmitter
A neurotransmitter that helps regulate mood, emotions, and behavior
is often targeted in the treatment of depression and anxiety disorders.
implicated in various psychological conditions and influences appetite, sleep, and cognitive functions.
Histamine (HA)
A neurotransmitter involved in immune responses, gastric acid secretion, and regulation of sleep-wake cycles, contributing to cognitive functions and allergic reactions.
ATP as a Neurotransmitter
A purine nucleotide that functions as a neurotransmitter involved in the modulation of pain, inflammation, and neuronal excitability
plays a key role in communication between neurons and can influence various physiological processes.
Adenosine as a Neurotransmitter
A neurotransmitter that promotes sleep, regulates blood flow, and inhibits neurotransmitter release
involved in reducing neuronal excitability and has a role in the sleep-wake cycle.
Endorphins
A group of neuropeptides that act as neurotransmitters, produced by the central nervous system
help to relieve pain and induce feelings of pleasure or euphoria, often referred to as the body's natural painkillers
mediate long-lasting psychomotor stimulation (e.g. restlessness, locomotion)
Enkephalins
A type of endogenous opioid peptide that acts as a neurotransmitter in the brain and spinal cord, playing a significant role in pain regulation and emotional responses
can bind to opioid receptors, providing analgesic effects similar to those of morphine
mediate rapid, transient effects (e.g. brief pain relief, vomiting)
Corticotropin-Releasing Factor (CRF/CRH)
A peptide hormone released by the hypothalamus that stimulates the release of adrenocorticotropic hormone (ACTH) from the pituitary gland
plays a crucial role in the stress response and regulates various physiological functions
Orexin
A neuropeptide produced in the hypothalamus that regulates arousal, wakefulness, and appetite
also involved in the pathophysiology of addiction and is associated with the reinforcement of drug-seeking behaviors.
Brain-Derived Neurotrophic Factor (BDNF)
A protein that supports the survival, development, and function of neurons, playing a crucial role in synaptic plasticity and cognitive functions
also involved in the reward circuit and is affected by drug exposure
binds to TrkB receptors on neurons
Vasopressin
A hormone produced in the hypothalamus that regulates water retention in the body and plays a role in social behavior, bonding, and stress response.
Oxytocin
A hormone and neuropeptide involved in social bonding, sexual reproduction, and childbirth
also plays a role in emotional responses and may influence substance use and addiction.
Anandamide
A neurotransmitter that binds to cannabinoid receptors, playing a role in pain regulation, appetite, mood, and memory
2-Arachidonoylglycerol (2-AG)
An endocannabinoid that acts on cannabinoid receptors, influencing various physiological processes such as appetite, pain sensation, and mood regulation.
Nitric Oxide (NO) as a Neurotransmitter
A gaseous neurotransmitter involved in various functions, including vasodilation, neuroprotection, and synaptic plasticity in the brain
Carbon Monoxide (CO) as a Neurotransmitter
A gaseous neurotransmitter that can modulate synaptic transmission and is involved in neuroprotection and signaling pathways within the brain.
Hydrogen Sulfide (H2S) as a Neurotransmitter
A gaseous neurotransmitter that plays a role in neuroprotection, modulation of synaptic transmission, and has potential effects on learning and memory processes.
Cholinergic Neurotransmitters
Chemical messengers that use acetylcholine as their primary neurotransmitter, involved in functions like muscle activation and memory
Axosecretory Synapse
A type of synapse where neurotransmitters are released directly from the axon terminal into the extracellular space/bloodstream, influencing nearby neurons without the need for direct synaptic connection.
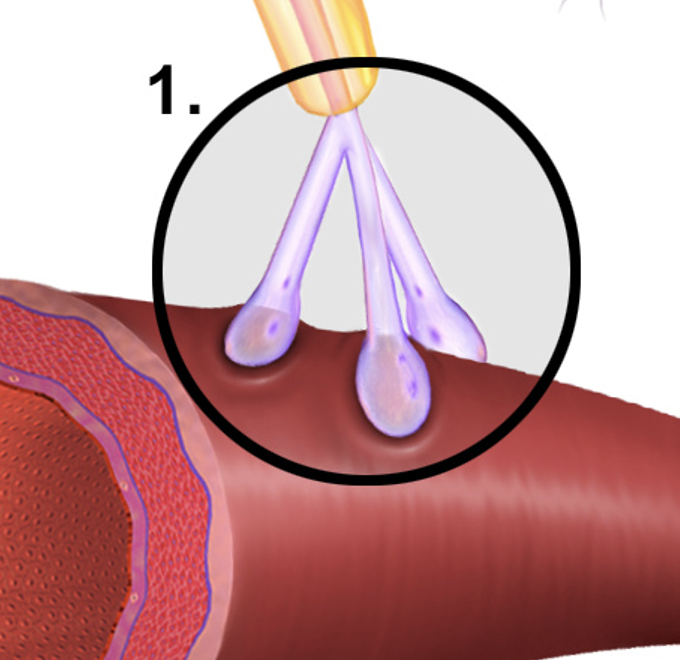
Axoaxonic Synapse
A type of synapse where one axon influences another axon's neurotransmitter release, thus modulating synaptic transmission between the connected neurons.
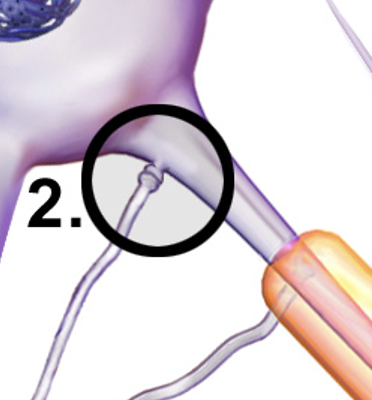
Axodendritic Synapse
A type of synapse where the axon terminal of one neuron connects to the dendrite of another neuron, enabling direct communication and neurotransmitter release.
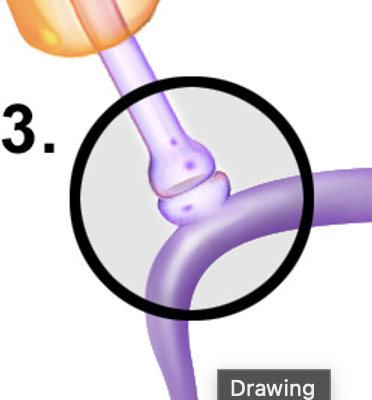
Axoextracellular Synapse
A type of synapse where neurotransmitters are released from the axon terminal into the extracellular matrix, affecting nearby cells without a direct connection.
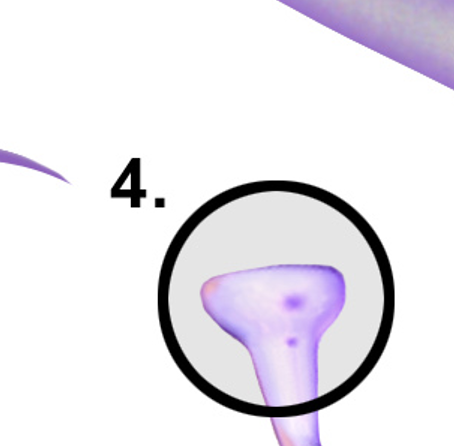
Axosomatic Synapse
A type of synapse where the axon terminal of one neuron connects to the soma (cell body) of another neuron, influencing its excitability and neurotransmitter release
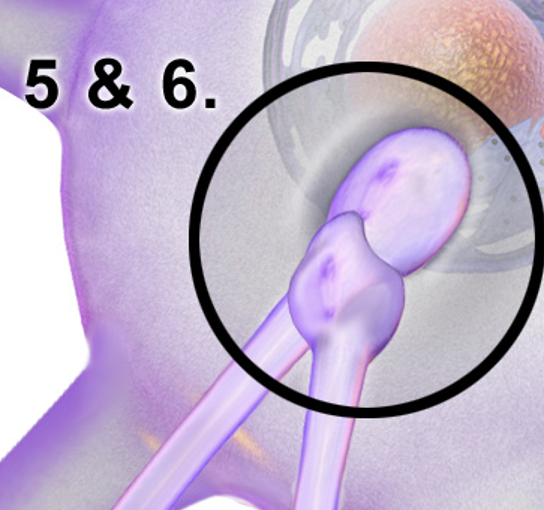
Axosynaptic Synapse
A type of synapse where the axon terminal of one neuron connects to the axon terminal of another neuron, regulating action potential propagation and neurotransmitter release.
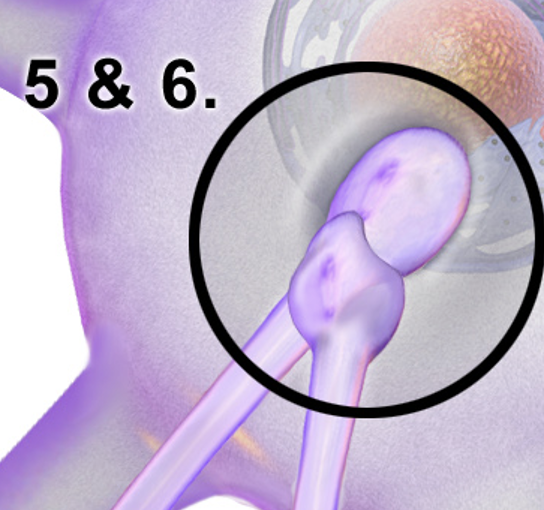
Readily Releasable Synaptic Vesicle
A type of synaptic vesicle that is primed and ready for release at the synapse, enabling fast neurotransmitter secretion in response to an action potential.
Reserve Pool Synaptic Vesicle
A type of synaptic vesicle that is stored away from the active zone of the synapse and can be mobilized for release during sustained neuronal activity.
Clathrin-Mediated Endocytosis (CME)
A cellular process utilized for the reuptake of synaptic vesicles after neurotransmitter release, ensuring the recycling of vesicles and maintenance of synaptic function.
Kiss & Run (K&R)
A synaptic vesicle recycling mechanism where vesicles partially fuse with the membrane to release neurotransmitters before quickly detaching and being recycled without prolonged membrane fusion.
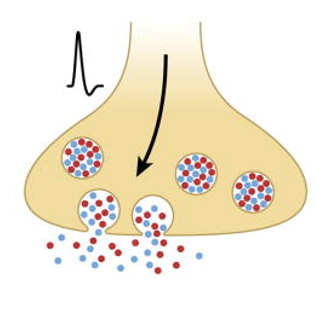
Co-Release of Neurotransmitters
The simultaneous release of two or more neurotransmitters from a single synaptic vesicle or from multiple vesicles, often leading to complex synaptic signaling and modulation of neuronal communication
NT’s are packaged into the same set of synaptic vesicles
upon an AP invading the presynaptic terminal, these vesicles are released
Co-Transmission of Neurotransmitters
A process in which two or more neurotransmitters are released simultaneously from the same neuron, allowing for enhanced or diversified signaling at the synapse.
this phenomenon enables more complex modulation of neuronal communication
requires NT’s to be sequestered into distinct populations of synaptic vesicles w/ release mediated by different Ca2+ sensitivities
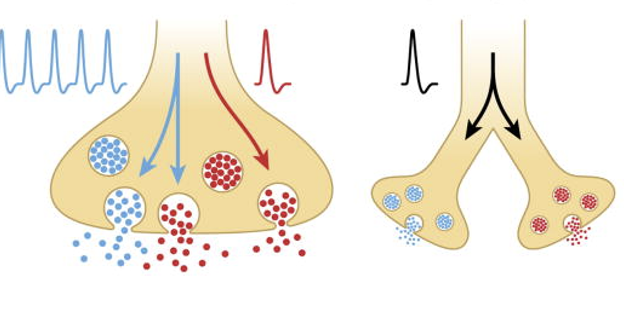
Spatial Segregation
The organization of neurotransmitter release and receptor distribution in such a way that different neurotransmitters are confined to distinct areas within the synapse, enhancing specificity in synaptic signaling and response
Ionotropic Receptors
A type of neurotransmitter receptor that, when bound by their ligand, open ion channels to allow ions to flow across the membrane, leading to rapid synaptic responses.
Metabotropic Receptor
A type of neurotransmitter receptor that, when activated by a ligand, initiates a signaling cascade through G-proteins, leading to slower and more prolonged synaptic responses.
Nerve Growth Factor (NGF)
A protein that supports the growth, maintenance, and survival of neurons, playing a crucial role in the development of the nervous system.
Catecholamines
A class of neurotransmitters that includes dopamine, norepinephrine, and epinephrine, which play key roles in mood regulation, attention, and the body's fight-or-flight response
derived from the amino acid tyrosine.
What kind of receptor are all dopaminergic receptors?
Metabotropic
D1 Dopaminergic Receptor
A subtype of metabotropic dopamine receptor that primarily stimulates adenylyl cyclase, increasing cAMP levels and influencing dopamine-related behaviors.
D2 Dopaminergic Receptor
A subtype of metabotropic dopamine receptor that primarily inhibits adenylyl cyclase, decreasing cAMP levels and modulating dopamine transmission.
What do varicosities along dopaminergic fibers represent?
Sites of DA neurotransmitter release in the brain, allowing for modulation of dopaminergic signaling throughout the neural circuit.
Termination of Action
The process by which a drug's effects are terminated or diminished, often through mechanisms such as metabolism, excretion, or receptor desensitization
What are the 2 primary means by which catecholamine action is terminated?
Degradation via enzymatic activity
Reuptake into the presynaptic terminal by catechol-O-methyltransferase (COMT) and monoamine oxidase (MAO)
Catechol-O Methyltransferase (CMOT)
An enzyme responsible for the degradation of catecholamines, such as dopamine and norepinephrine, by transferring a methyl group