Atoms section 1 quiz:
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
36 Terms
Elements aka
Atoms
What are atoms?
The smallest unit of matter that all known objects are made of.
In a small pile of carbon there are approximately:
602 septillion( 1 mole)
One Mole(Avagadros Number)
602 septillion atoms
What are atoms made up of?
Nothing, subatomic particles
Protons: charge? Location? Mass?
Positive, nucleus, 1 AMU ( atomic mass unit)
Neutron:charge?location? Mass?
Neutral, nucleus, 1 AMU
Electron: charge? Location?mass?
Negative, orbiting nucleus, 0 AMU
How do we determine the atomic mass?
Add the number of protons and neutrons ( add their masses) this is because Electrons don’t weigh basically anything.
In the P-table the atomic mass is an….
Average( round the number to nearest whole) (effects the number of neutrons)
How do we determine the number of neutrons?
Atomic wight(rounded) - atomic number( protons/electrons)
Number of electrons=
Atomic number or number of protons
Electron orbitals or…
Lobes, shells, or rings
Lobes, shells, and rings are:
Places where electrons can be found
First 4 orbitals:
K,L,M,N
Electrons cannot exist in the next orbital unless…
Until the preceding orbital is maxed out with electrons.
Max of K orbital:
2
Max of L Orbital:
8 electrons
Max of M
8 ( or 18 but mainly 8 for this class)
Bohr Model:
A model of an element that shows where each electron is on each orbital.
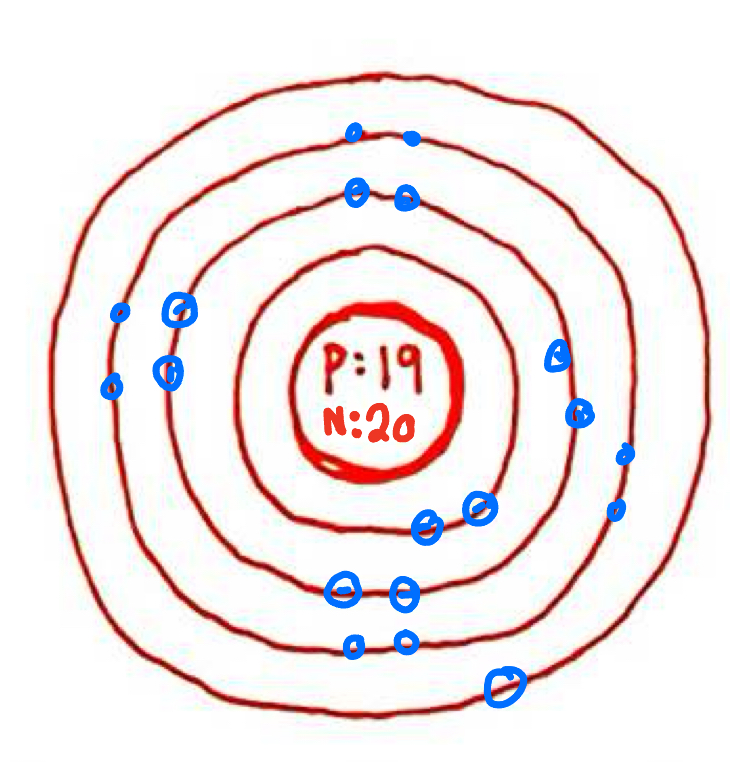
What is the configuration of these electrons?
2-8-8-1
Valence electrons:
Electrons that are located on the last orbital
Ex: oxygen has an atomic number of 8, how many valence electrons does it have?
2-6, it has 6 valance electrons
The maximum number of valance electrons is 8 For all elements except…..
Hellium+ Hydrogen, their max is two because they only have up to two electrons.
The periodic table is ordered by:
Amount of Valance electrons.
Lewis dot structure only shows the…
Valance electrons.
As you go down a family(vertically down)
The amount of orbitals increases
As you go across a period( the colors horizontally)
The number of valance electrons increases.
Group 8a has
Max valance electrons
Family 1a is named:
Alkali metals
Family 2a is named
Alkaline Earth Metals
Groupd 7a is named:
Halogen
Group 8a is named:
Noble Gases
Families are ordered by:
number of valance electrons.
Periods are ordered by:
Number of orbitals