Oxygen dissociation
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
What is haemoglobin
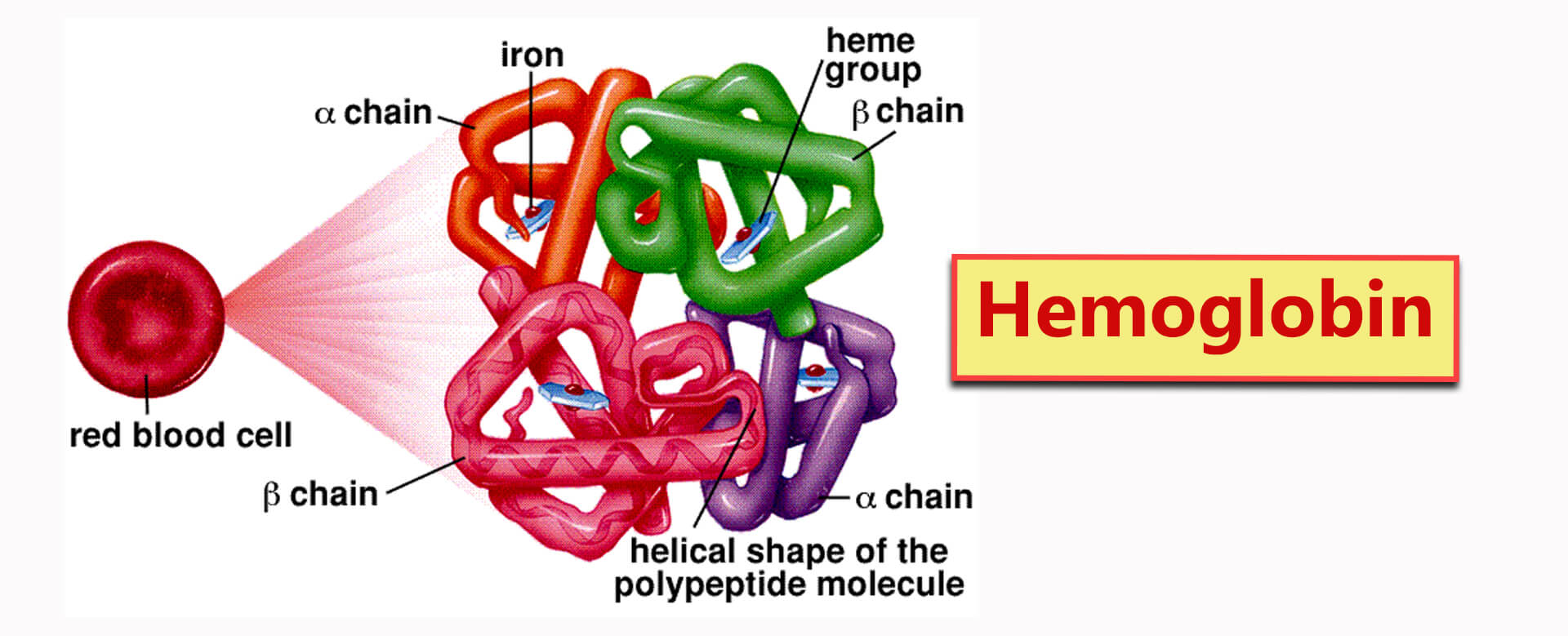
Haemoglobin is a water soluble gobular protein that has a quaternary structure. This means that it consists of more than one polypeptide chain.
Two chains are ⍺-polypeptides (alpha) and two chains are β-polypeptides (beta).
each of the polypeptide chains are associated with a haem group. Each haem group contains an Fe2+ ion which can combine with an oxygen molecule (O2).
Each haemoglobin molecule can therefore carry four oxygen molecules.
What is the process of the transport of oxygen by haemoglobin ( explanation for my understanding)
The association (or loading) of oxygen is the process by which haemoglobin binds with oxygen. In humans, oxygen association occurs in the lungs.
After oxygen association, the red blood cells transport the oxygen. Oxygen is transported from the lungs to the rest of the body.
The dissociation (or unloading) of oxygen is the process by which oxygen is released from haemoglobin. In humans, oxygen dissociation occurs at cells which require oxygen, where haemoglobin returns to the lungs in order to bind to oxygen again.
Affinity is the degree to which one substance combines with another. Haemoglobin has different affinities for oxygen molecules under different conditions.
When oxygen concentration is high, haemoglobin has a high affinity for oxygen. This means that it will readily associate with oxygen and will dissociate with it less easily.
When oxygen concentration is low, haemoglobin has a low affinity for oxygen. This means that it will readily dissociate with oxygen and will associate with it less easily.
Haemoglobin changes its affinity for oxygen by changing its shape when in the presence of certain substances. For example, in high carbon dioxide (CO2) concentration, haemoglobin has a low affinity for oxygen, whereas in low CO2 concentration, haemoglobin has a high affinity for oxygen.
Having changing affinities for oxygen means that oxygen is only associated/dissociated where necessary. This makes haemoglobin efficient at transporting oxygen because oxygen is readily associated at the gas exchange surface (the lungs) and readily dissociated at the tissues which need it.
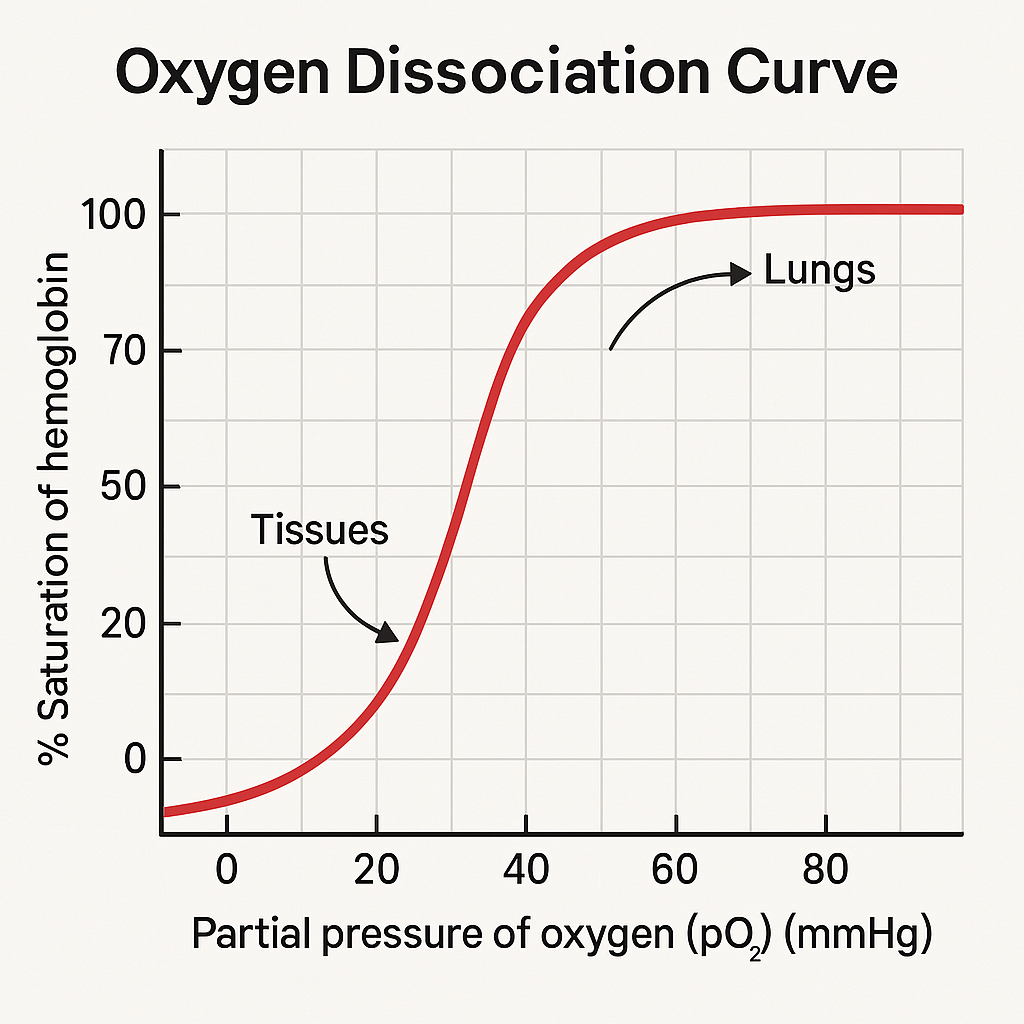
Oxyhaemoglobin dissociation curves
The partial pressure of oxygen is a measure of oxygen concentration. Partial pressure is measured in kilopascal (kPa).
The greater the concentration of dissolved oxygen in a cell, the greater the partial pressure.
Haemoglobin has different affinities for oxygen depending on its partial pressure. Haemoglobin will readily associate more tightly with oxygen if the partial pressure of oxygen is high and will readily dissociate with oxygen if the the partial pressure of oxygen is low. These two processes are known as loading and unloading.
The process of respiration uses up oxygen. This decreases partial pressure, in turn decreasing affinity of oxygen for haemoglobin. As a result, oxygen gets released to respiring tissues where needed.
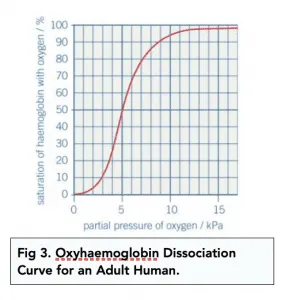
Oxyhaemoglobin dissociation curves are S-shaped. They show the relationship between the partial pressure of oxygen and the saturation of haemoglobin with oxygen.
Saturation can have an effect on affinity.
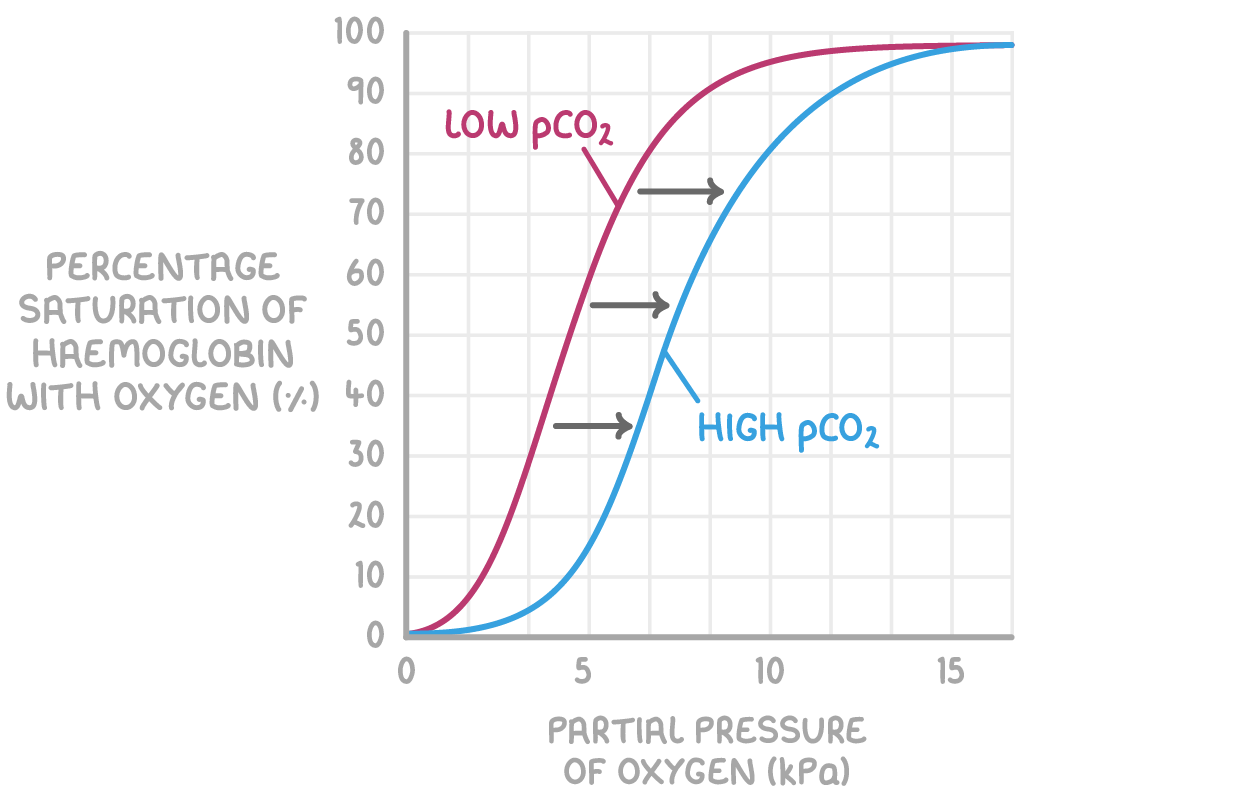
What is the Bohr effect
pCO2 affects haemoglobin saturation as follows:
Higher pCO2 at respiring tissues causes haemoglobin to release oxygen.
This is because of the Bohr effect, which is the decreased affinity for oxygen in haemoglobin when carbon dioxide is present.
This means the oxygen saturation of haemoglobin is lower for a given pO2 when pCO2 is higher.
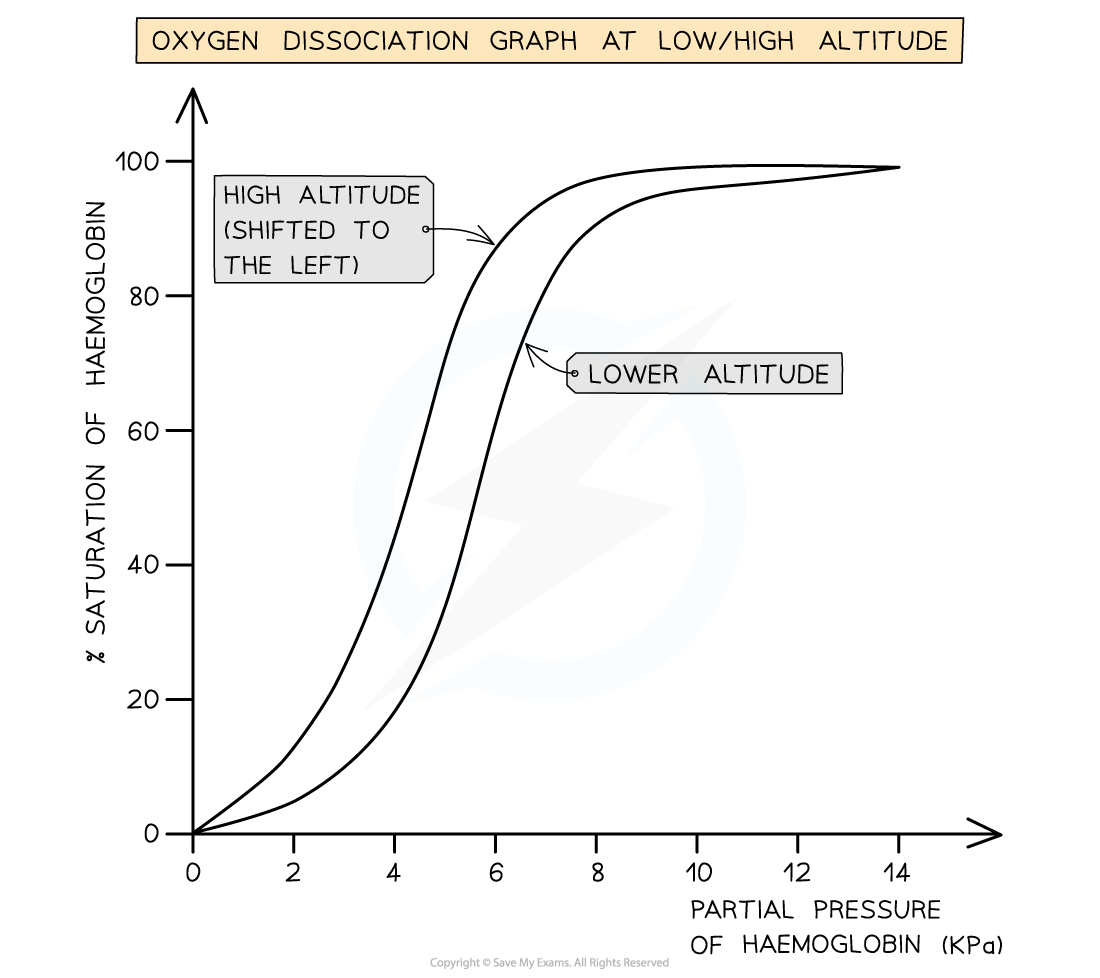
what does the ODC of a shrew look like and explain
Shrew is a small mammal so larger SA:Vol, so has rapid heat loss
High metabolic rate to replace lost heat requires O2
Lower affinity for O2, so is better at unloading oxygen at respiring cells
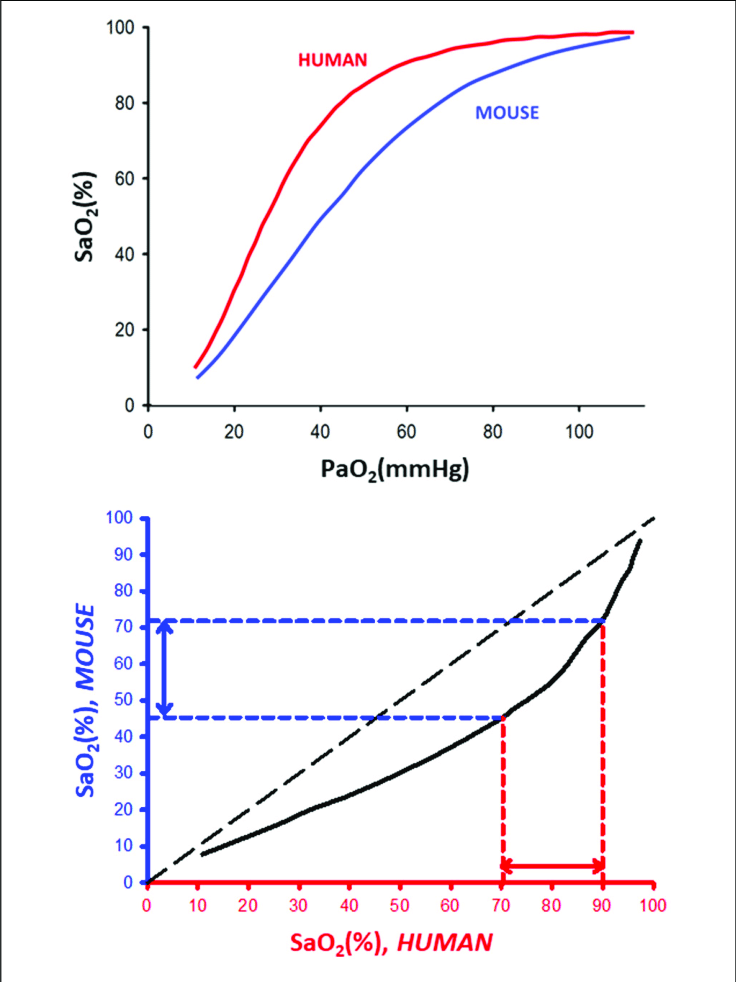
what does the ODC of a llama and other high altitude animals look like and explain
Higher affinity for O2 because they live in areas of lower pO2
Better at loading O2 in lungs so curve is shifted to the left
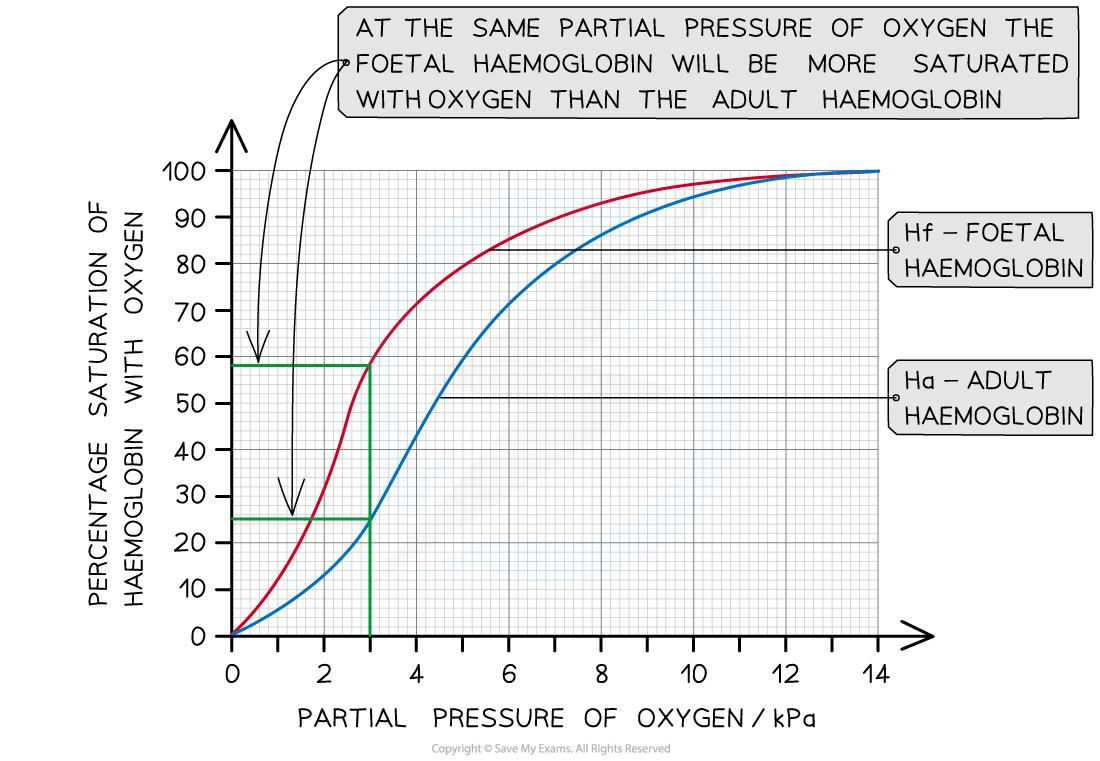
What does the ODC for feotuses look like
Fetal haemoglobin differs from adult haemoglobin because:
The fetus needs to obtain oxygen from the mother's blood.
The fetal haemoglobin therefore has a higher oxygen affinity than the adult haemoglobin found in the mother's blood.
This allows the oxygen to dissociate from the mother's haemoglobin, and bind with haemoglobin in the fetal blood.
This ensures that the fetus gets enough oxygen to survive while it develops.
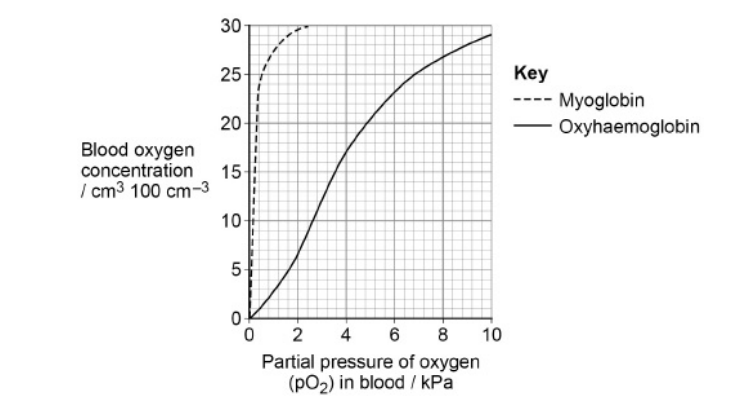
Seals are diving mammals. They fill their lungs with air before they dive and hold their breath during the dive. The graph shows the dissociation curves for seal oxyhaemoglobin and seal myoglobin. Myoglobin is an oxygen-carrying protein found in muscles.
Use information in the graph to explain how the seal’s myoglobin dissociation curve shows the seal is adapted for diving.
1. High(er) affinity for O2 (than haemoglobin) OR Dissociates oxygen less readily OR Associates more readily;
2. Allows (aerobic) respiration when diving/at low(er) pO2 OR Provides oxygen when haemoglobin unloaded OR Delays anaerobic respiration/lactate production;
Give two safety precautions that should be followed when dissecting a heart.
Wash hands/wear gloves Disinfect bench/equipment Cover any cuts Cut away from self/others/on a hard surface Safe disposal/Use a sharp scalpel/scissors
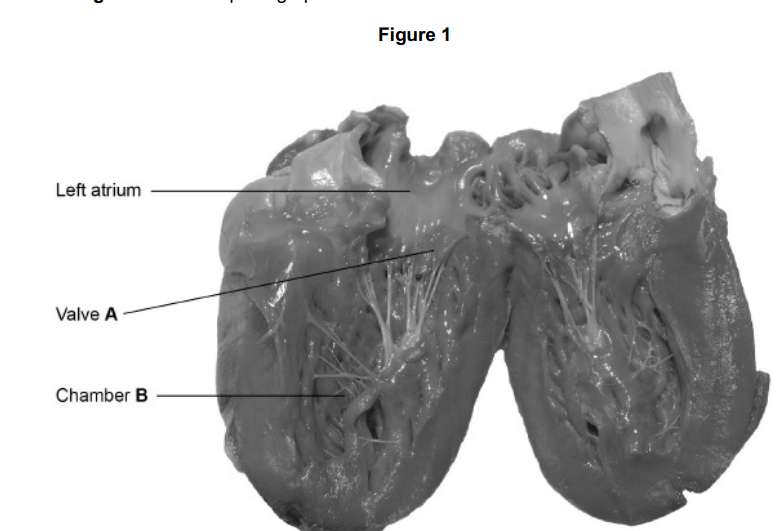
Name valve A and chamber B.
Valve A - (Left) atrioventricular
Chamber B- Left ventricle
Explain how valve A in Figure 1 maintains a unidirectional flow of blood.
Pressure in (left) atrium is higher than in ventricle/B causing valve to open;
Pressure in (left) ventricle/B is higher than in atrium causing valve to close