Photoelectron spectroscopy (1.6)
0.0(0)
0.0(0)
Card Sorting
1/6
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
7 Terms
1
New cards
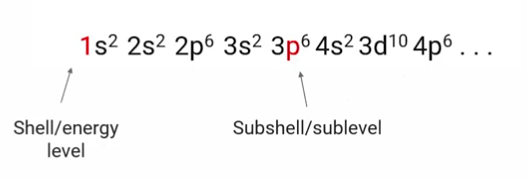
electron configuration
electrons exist in “shell(energy levels)” and subshells(sublevels)”
outer electrons are valence electrons
outer electrons are valence electrons
2
New cards
Aufbau Principle
means “to build up”
electrons fill lower energy levels first and then higher ones
is determined by photoelectron spectroscopy
electrons fill lower energy levels first and then higher ones
is determined by photoelectron spectroscopy
3
New cards
photoelectron spectroscopy (PES)
determines the relative energies of electrons in atoms/ions
use radiation to remove an electron form an atom
valance(outer) electrons are easier to remove than core(inner) electrons
use radiation to remove an electron form an atom
valance(outer) electrons are easier to remove than core(inner) electrons
4
New cards
photoelectron spectroscopy process
1)Irradiation: Sample is exposed to high-energy photons (usually X-rays or UV light).
2)Photoejection: Electrons are ejected from the sample's outermost energy levels.
3)Detection: Energies of emitted electrons are measured and analyzed.
2)Photoejection: Electrons are ejected from the sample's outermost energy levels.
3)Detection: Energies of emitted electrons are measured and analyzed.
5
New cards
photoelectron spectroscopy peaks
correspond to different energy levels of electrons in an atom
the position and intensity of the peaks can be used to differentiate between different elements
the position and intensity of the peaks can be used to differentiate between different elements
6
New cards
take note
rewatch the second ap daily 1.6 video for VERY helpful example problems!!!!
7
New cards
Binding energy
the energy needed to KEEP an electron in an atom (different from ionization energy which is the energy needed to REMOVE an electron)