genetic determinants of pharmacokinetic variability
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
19 Terms
variable response across populations
same diagnosis, same drug, same dose →
drug effective and harmful
drug not effective and harmful
drug effective and not harmful
drug not effective and not harmful
other factors that influence treatment
ethnicity
age - fat distribution is different at different ages
pregnancy - cardiac output is increased, higher filtration rate (drug eliminated at a faster rate), fat distribution is different
disease
drug/food interactions - grapefruit interacts with CYP3A4 → increase in metabolism
PK/PD interactions
genetic variation
pharmacogenetics
the effect of genetic variants on the response to drugs
normally focused on a single drug, single gene and/or single drug response
pharmacogenomics
the effect of the persons genome on response to drugs
normally looks at the entire genome of an individual, taking into consideration the combined effect of multiple variants and multiple drugs
drug variability
therapeutic window - the range of drug concentrations in the blood that provides effective treatment without causing toxicity
e.g. drugs with a narrow therapeutic index, its hard to find a balance where the drug can cause therapeutic effect and not cause toxicity
reasons for drug variability
patient genetic variability can influence how the individual responds to certain treatment
pharmacokinetics: ADME
pharmacodynamics: receptors, ion channels, enzymes
ADME
absorption: how the drug gets into the body and circulation
distribution: how the drug is dispersed throughout the body
metabolism: how the drug is transformed (activated or inactivated)
excretion: how the drug is removed from the body
control of ADME
transporters are key for the absorption, distribution and excretion of drugs
transport drugs across biological membranes using wither ion gradients or ATP
key in the bowel, kidneys, BBB and liver (and placenta in pregnacy)
ATP binding cassette (ABC)
solute carrier proteins (SLC) - OCTs and OATs
prone to genetic variation
e.g. ABCB1 - produces P-gp, transports loads of hydrophobic substrates
metabolism
phase 1 - functionalisation reactions (CYP450), introduces a functional group on the drug to make it more polar and prepared it for phase 2
phase 2 - conjugation reactions, attaches polar group to increase water solubility for excretion
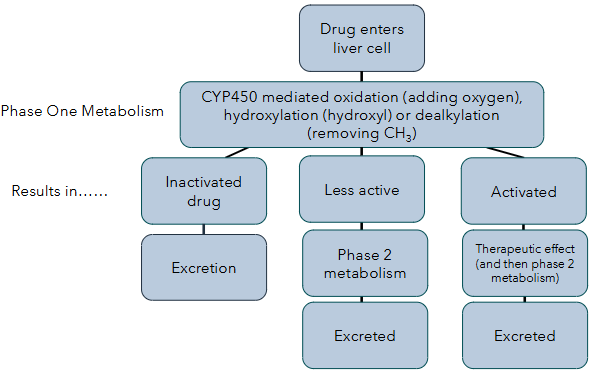
cytochromes
mostly found in the liver
process around 75% of drugs
have a haem-iron group at the active site
can be affected by loss of function and gain of function mutations
how cytochromes work (phase 1)
drug binding → haem iron is reduced from 3+ to 2+ by electrons, oxygen binds which activates it → oxygen splitting and transfer, one oxygen atom is inserted into the substrate and the other is reduced to water → product release, oxidised product is released and the enzyme returns to its resting state
codeine
morphine prodrug, activated by CYP2D6 enzyme
used to treat mild-moderate pain
MOA: decreasing pre-synaptic release of neurotransmitters → decreasing post-synaptic neuronal excitability → promoting descending inhibition
CYP2D6 gene is highly polymorphic e.g. missense and frameshift mutations → variations in activity
phenytoin
anti-epileptic drug
narrow therapeutic window
metabolism carried out by CYP2C9 and CYP2C19 - has two missense polymorphisms that reduce metabolism
phase 2 enzymes
UGTs - glucuronidation
SULTs - sulphation
DPYD - dihydro group to pyrimidines
GSTs - glutathione
NATs - acetylation
TPMT - methylation
5-fluorouracil
chemotherapeutic used to treat many cancers
anti-metabolite - resemble natural metabolites and interfere with DNA or RNA synthesis by inhibiting thymidylate synthase (TS)
sometimes cause severe side effects due to toxic build up of the drug in the blood
metabolism - inactivated by phase 2 enzyme DPD
affected individuals had functional variants int he gene that resulted in formation of inactive or decreased functional protein
irinotecan
topoisomerase 1 inhibitor used to treat colorectal and pancreatic cancer
prodrug that is converted to its active metabolite, SN-38
SN38 is inactivated by UGT enzyme encoded by the UGT1A1 gene
most common variant is an insertion mutation → reduced gene transcription and activity → irinotecan toxicity as the active metabolite cannot be cleared
genetic variability - pharmacodynamics (trastuzumab)
HER2+ RTK amplification in breast cancer → increased cancer cell proliferation, growth and survival via amplification of MAPK and PI3K/Akt signalling pathways
challenges with pharmacogenetic profiling
ethnic variations
cost
availability of testing
drug type