Cell Cloning Part 3 - 5
1/69
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
70 Terms
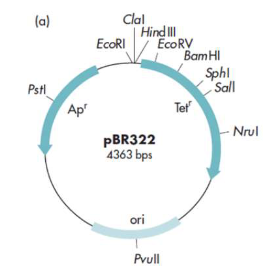
Steps of cloning (YFG) using pBR322
Must use PstI to cut your gene and plasmid
Cut plasmid and DNA using PstI
Use DNA ligase to from the recombinant plasmid, Am gene non-functional
Transform the plasmid into the E. coli cells
Spread the cells onto a plate with tet. and incubate overnight to eliminate the cells with no plasmids
Using replica plating, spread colonies onto separate agar plates containing Ampicillin
Any of the colonies, which do not grow on the agar containing tetracycline, can be recovered from the ampicillin plate (master plate)
Alkaline Lysis
Alkaline Lysis
The most straightforward and reliable techniques for purification of plasmid DNA
A quick method
This procedure is often described as a “miniprep”
It yields about 1 μg of DNA, which is enough for many analytical purposes
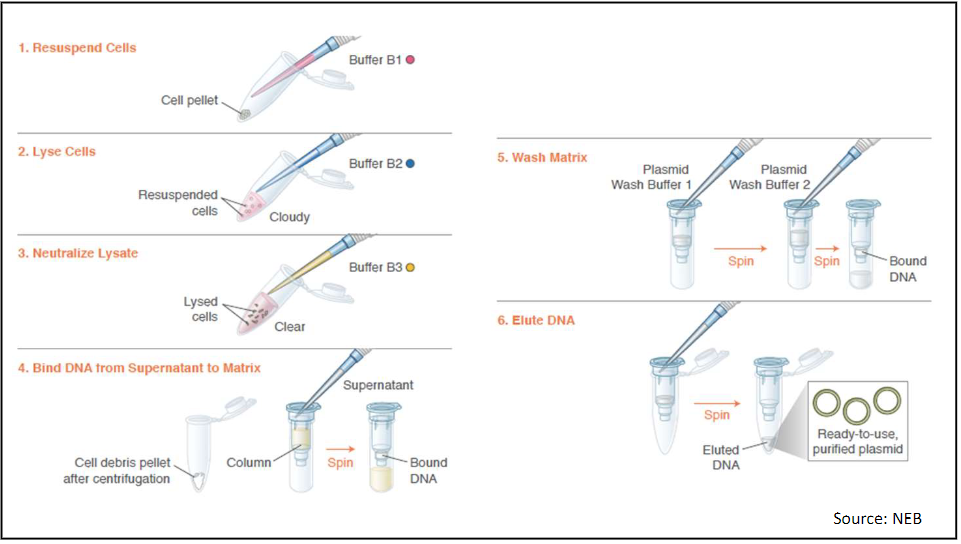
What produces protein
Give the bacteria a chemical signal that instructs them to make the target protein
The bacteria serve as miniature “factories”
e.g. Human insulin
Expressing human gene in bacteria
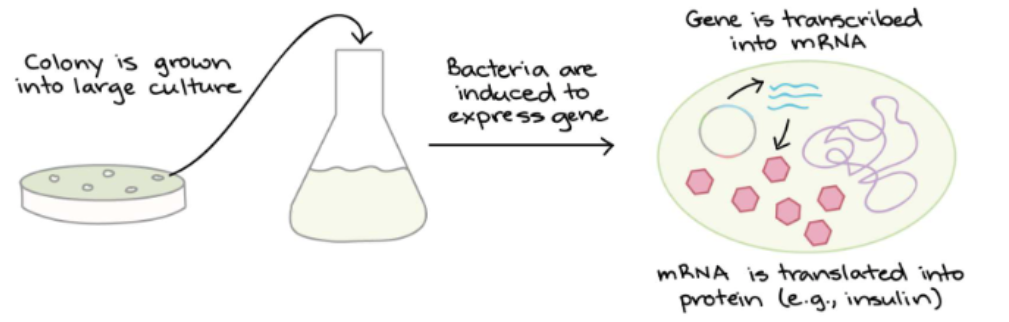
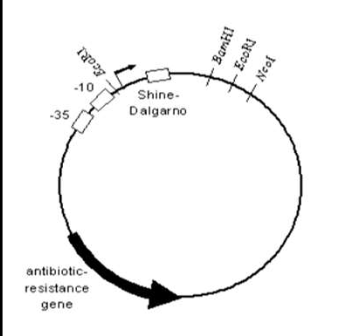
Shine Dalgarno
Plays a role in initiation of translation (ribosomal binding site)
Is it important that the enhancer and TATA sequence might be left behind due to the placement of the Shine Dalgarno?
No
The plasmid provides the needed reeded regulatory signals
Restriction Mapping
The process of obtaining structural information on a piece of DNA using restriction enzymes
Used to map an unknown segment of DNA
Used to analyze recombinant closes
after purifying the plasmid DNA from individual clones
Cut plasmid with restriction enzymes, and analyze the sizes of the fragments produced
Plasmid Structural info
Size of plasmid
Restriction sites
Steps of restriction mapping
Cut the plasmid DNA with various restriction enzymes
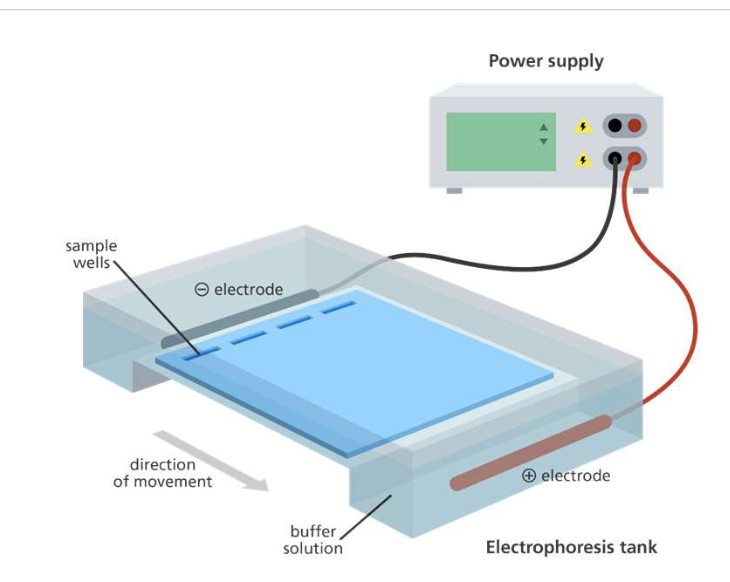
Run Gel Electrophoresis
Analyze the sizes of the fragments produced to obtain structural information
Gel Electrophoresis
To separate the desired DNA based on size after using the restriction enzymes
DNA: Negatively charged (phosphate backbone)
DNA molecules are separated according to their size in a gel
Actual size is estimated by comparison with marker DNAs of known size
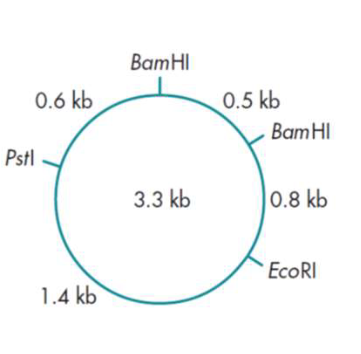
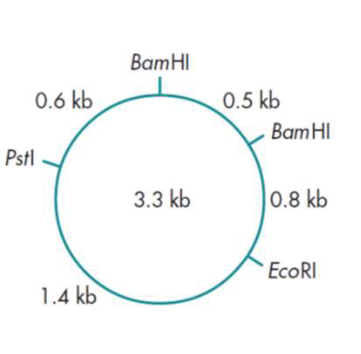
What would be the sizes of the fragments that you would expect to find if you
cut this plasmid with (a) BamHI, (b)EcoRI, (c) both BamHI and EcoRI together?
(a) If you cut with BamHI you would expect two fragments of 0.5 kb and 2.8 kb.
(b) If you cut with EcoRI you would expect a single fragment of 3.3 kb.
(c) If you use both restriction enzymes together you would expect three fragments of 0.5, 0.8 and 2 kb.
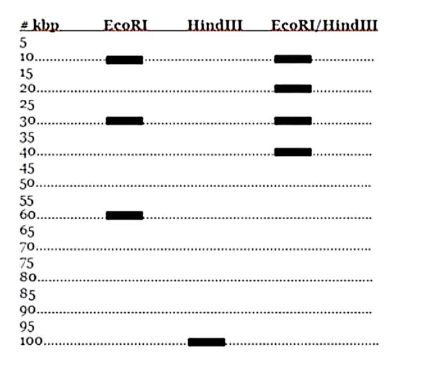
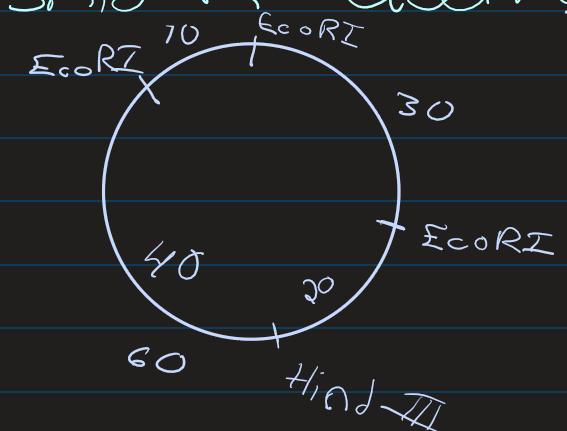
Create a plasmid map using the following electrophoresis data
See that there are 3 EcoRI (1st column)
1 10kbp
1 30kbp
1 60 kbp
See that there is 1 HindII (2nd conlum)
Split the 60kpd section into: (3rd column)
20kpd
40kpb
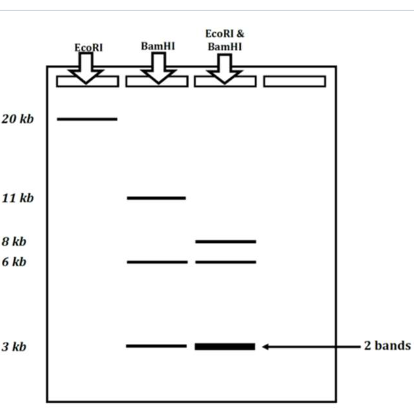
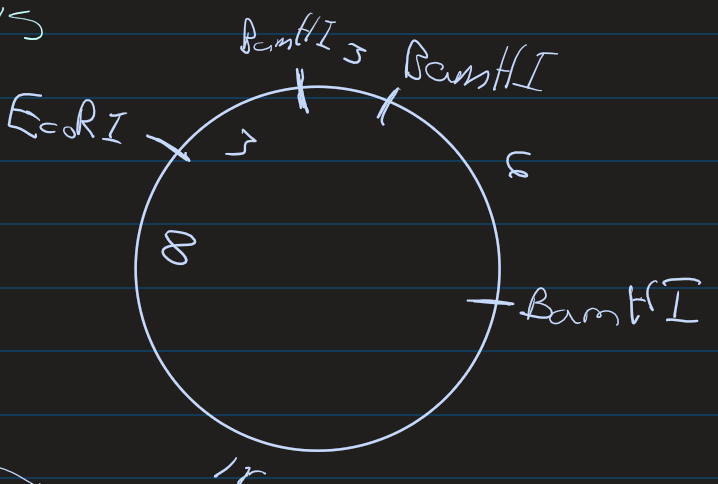
Create a plasmid map using the following electrophoresis data
1st column tells us
1 EcoRI
2nd column tells us
3 BemHI
1 11kb
1 6 kb
1 3 kbp
3rd column tells us
The EcoRI splits the 11kd section
3 kb
8 kb
Applications of Recombinant DNA Technology
Medical Applications
Agricultural Applications
Industrial Applications
Medical Applications of Recombinant DNA Technology
Gene therapy
Production of transgenic organisms
Diagnosis of genetic disease
Gene Therapy
Primarily an experimental technique that uses genes to treat or prevent disease (NIH)
Is Gene Therapies FDA approved
The US FDA has approved a total of 34 cellular and gene therapy products within the US
LUXTRNA (2017), Spark Therapeutics, Inc
First FDA approved gene therapy product
Recombinant AAV2 delivers RPE65 to treat patients with RPE65-mutation-associated retinal dystrophy (inherited).
LYFGENIA (2023), Bluebird Bio, Inc
Cell (autologous, modified): Hematopoietic stem cells engineered with a lentivirus, BB305, expressing modified ßA-globin gene.
Treats patients with certain kinds of sickle cell disease
CASGEVY (2023), Vertex Pharmaceuticals, Inc
Cell (autologous, modified): CD34+ hematopoietic stem cells engineered with electroporation of CRISPR/Cas9 RNP complexes to decrease expression of BCL11A to increase fetal hemoglobin production.
Treats patients with certain kinds of sickle cell disease.
Approaches to Gene Therapy
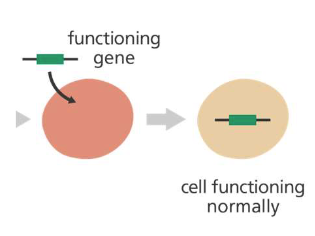
Inactivate a mutated gene that is functioning improperly (Gene inhibition therapy)
Replace a mutated gene that causes disease with a healthy copy of the gene
Introduce a new gene into the body to help fight a disease
Gene inhibition Therapy
Inactivate a mutated gene that is functioning improperly
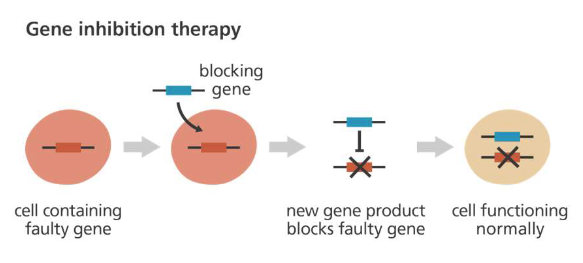
Gene replacement therapy
Replace a mutated gene that causes disease with a healthy copy of the gene
Gene introduction therapy
Introduce a new gene into the body to help fight a disease
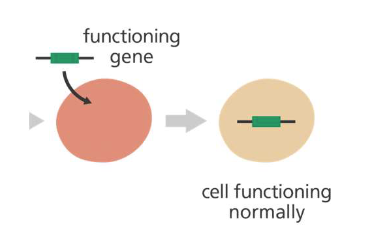
Gene Therapy Procedure
A vector is genetically engineered to deliver the gene (viruses)
The viruses are modified so they can't cause disease when used in people
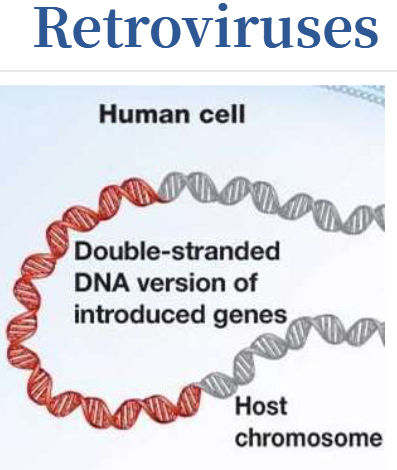
Retroviruses
Integrate their genetic material (including the new gene) into a chromosome in the human cell
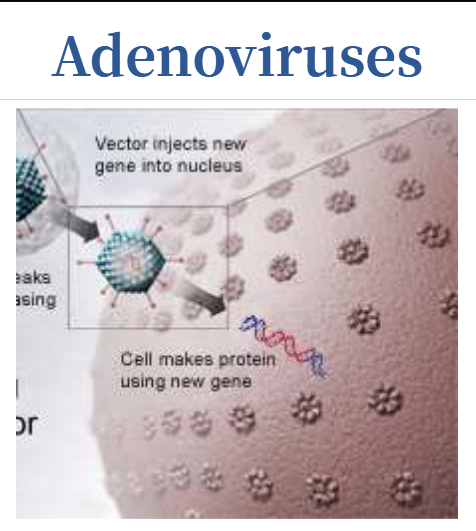
Adenoviruses
Introduce their DNA into the nucleus of the cell, but the DNA is not integrated into a chromosome
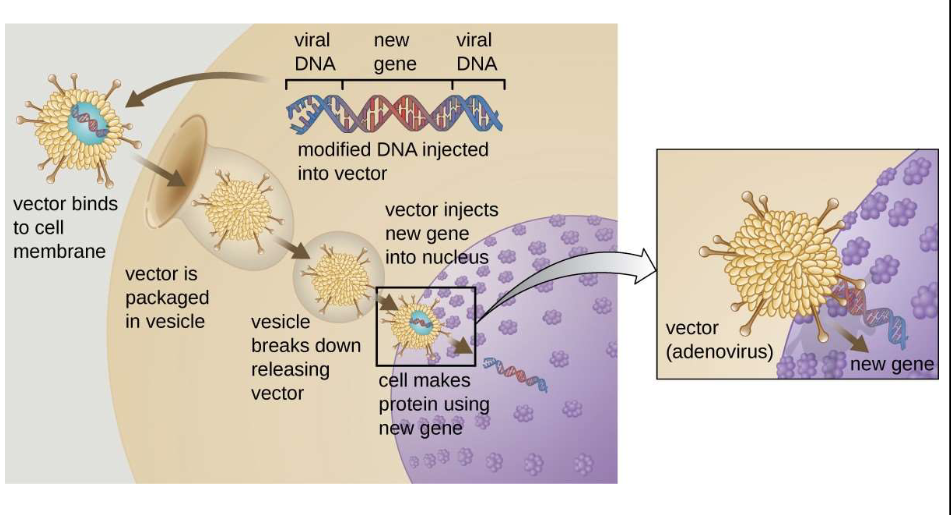
Adenovirsus Procedure
Vector can be injected or given intravenously (IV) directly into a specific tissue in the body, where it is taken up by individual cells
A sample of the patient's cells can be removed and exposed to the vector in a laboratory setting
The cells containing the vector are then returned to the patient
Applications of Gene Therapy
A promising treatment option for a number of diseases
Cancer, cystic fibrosis, heart disease, and diabetes
Limitations of Gene Therapy
Remains an area of active research and study
long-term effects under investigation
there are risks
Risks of Gene Therapy
Unintended Immune Responses
Off-Target Effects
Targeting the wrong cells
Unintended locations in the genome
Infection caused by the virus.
Risk of Tumorigenesis
Ethical & Social Concerns
Unintended Immune Responses
The body may see the newly introduced therapeutic gene or the vector used to
deliver it as intruders and attack the. This may cause inflammation and, in severe
cases, organ failure.
Off-Target Effects - Targeting the Wrong Cells
Because viruses can affect more than one type of cells, it's possible that the altered viruses may infect additional cells — not just the targeted cells containing mutated genes. If this happens, healthy cells may be damaged, causing other illness or diseases, such as cancer.
Off-Target Effects - Unintended locations
The possibility of the therapeutic gene integrating into unintended locations in the genome, potentially disrupting normal gene function
Infection caused by the virus
Viral Replication: It's possible that once introduced into the body, the viruses may recover their original ability to replicate to cause disease
Risk of Tumorigenesis
Possibility of causing a tumor: Insertional mutagenesis, where the integration of the therapeutic gene into the genome increases the risk of tumor formation.
Types of Gene Therapy
Somatic Therapy
Germ-line Therapy
Somatic Therapy
Healthy copy of a gene is inserted into somatic cells
Affects only the targeted cells in the patient
not passed on to future generations
Germ-line Therapy
Treats cells that make eggs and sperm
Permanent changes that are passed down to subsequent generations
The goal would be to change the eventual child's genetic inheritance
Ethical Issues
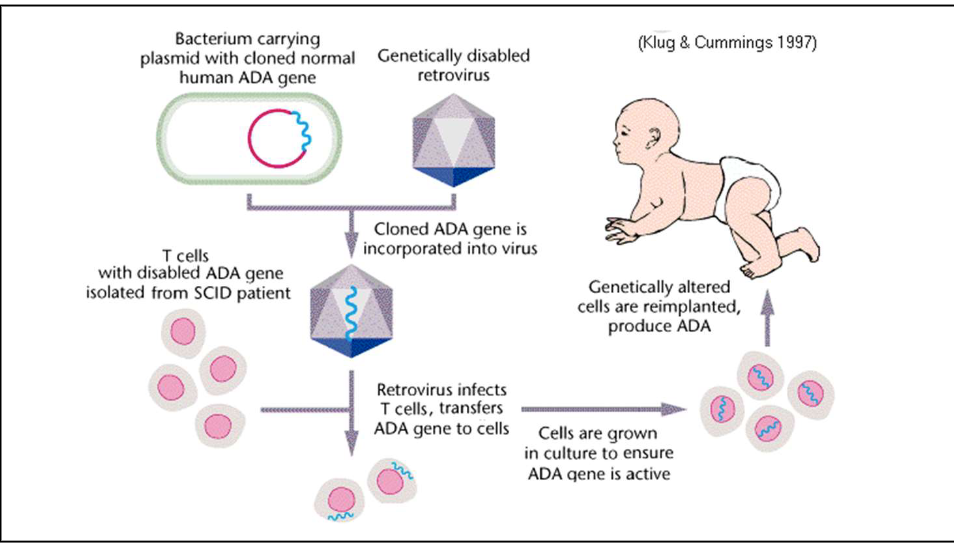
First Human Gene Therapy Experiment
Carried out at NIH in 1990
The trial aimed to treat a genetic disorder called severe combined immunodeficiency (SCID)
Specifically the form caused by a deficiency of the adenosine deaminase (ADA) enzyme. The condition is commonly known as ADA-SCID.
the initial results showed some improvement in immune function
the overall success was limited, and the treatment did not provide a complete cure.
this groundbreaking experiment marked the beginning of human gene therapy research.
Production of Transgenic Animals
Recombinant DNA technology has made it possible to create transgenic animals to produce human proteins
Transgenic Animals
Animals that are genetically modified
Important tools for researching human disease
Mice have been genetically modified to naturally produce human antibodies for use as therapeutics
Diagnosis of Genetic Disease
Can provide precise diagnostic information about genetic diseases, allowing appropriate counselling, and indicating future directions for research on therapeutic intervention, e.g. gene therapy.
Genetic screening
Genetic sequencing
Agricultural Applications of Recombinant DNA Technology
Improving yields
Resistance to disease
Way of using recombinant DNA technology to improve yields
A faster rate of growth
An increase in the quantity of the food produced by plants
Nitrogen Fixing Gene
N2 is vital nutrients for most plants
Plants are unable to utilize N2, which is abundant in the atmosphere
Fixation
Synthetic nitrogen fertilizer, Problems
Solution
N2 Fixation
Convert N2 to a useable form like ammonia
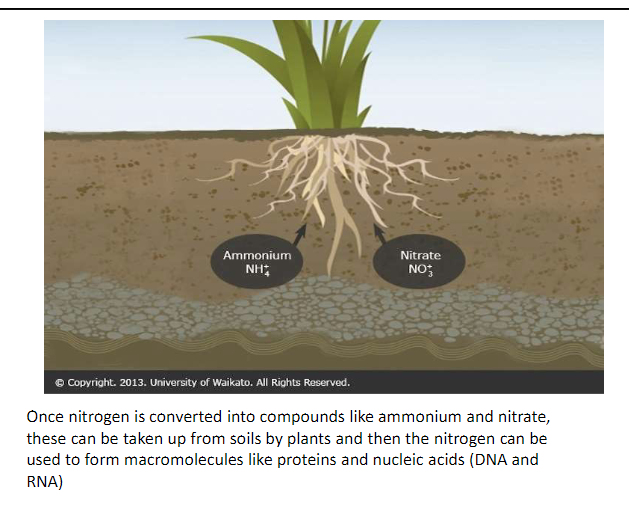
Synthetic Nitrogen Fertilizer
Biologically reactive nitrogen is therefore routinely supplied to crops as synthetic nitrogen fertilizer
Synthetic Nitrogen Fertilizer Problems and Solution
The extensive use of synthetic fertilizer in agriculture has substantial environmental costs
Synthetic fertilizers lead to pollution
Many of these problems could be avoided if plants could be engineered to fix nitrogen directly from air
Procedure to make plants fix nitrogen from the air
Nitrogen fixing genes can be cloned in E. coli and placed into cells in crop
This would enable these crops to fertilize themselves effectively, saving much money and increasing yields
How to make crops resistance to disease
Genes are introduced into crops, causing them to produce their own pesticides
Example of making crops resistance to disease
Place a gene into tomato plant that produces a protein, which kills tomato worms
When modified tomato plants and normal plants were grown together in a laboratory and deliberately infected with worms
The normal plants were stripped of their leaves, but the modified plants were unaffected
Industrial Application of Recombinant DNA Technology
Bioprocessing
Production of Drugs and Food
DNA Sequencing
The Polymerase Chair Reaction (PCR)
PRC
Polymerase Chair Reaction
An in vitro technique for synthesizing many copies of DNA
Generates thousands to millions of copies of a particular DNA sequences
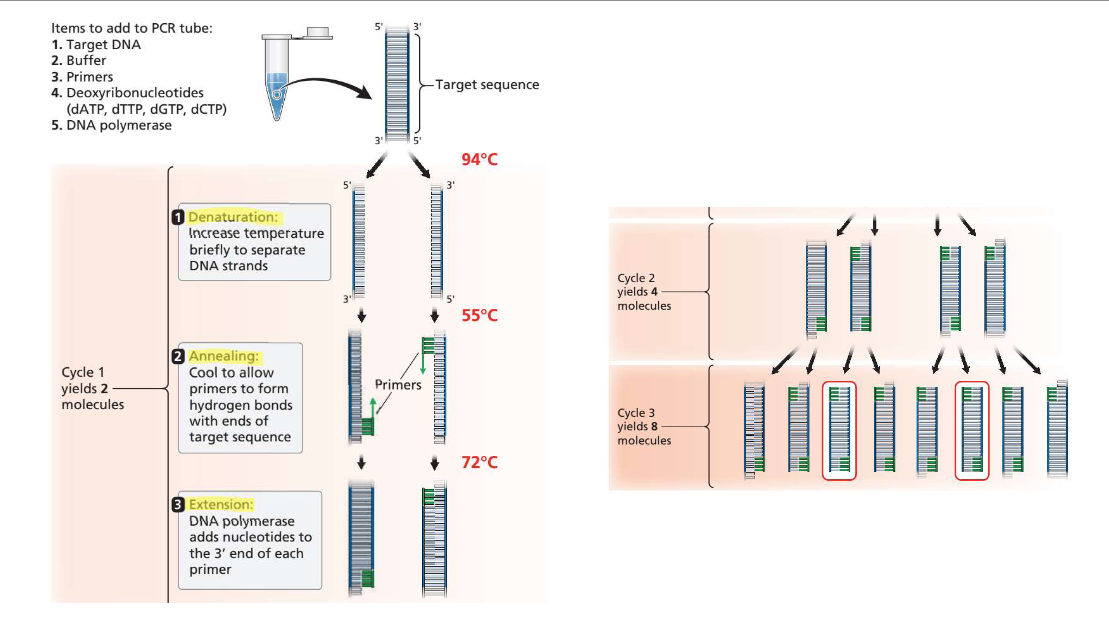
PCR-Required Materials
DNA polymerase
A single-stranded DNA template
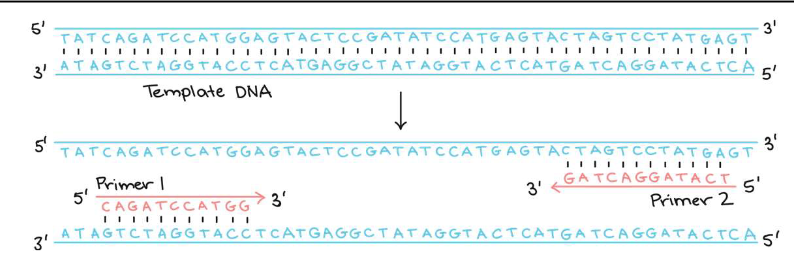
PCR Primers
Deoxynucleotide triphosphates (dNTPs)
DNA Template (for PCR)
A nucleotide sequence that directs the synthesis of a sequence complementary to it
Taq DNA polymerase
Enzyme that copies the DNA
PCR Primers
A strand of DNA bases that enables DNA to be replicated

dNTPs
Single units of the bases A, T, G, and C,
Building blocks for new DNA strands
First step of PCR
PCR primers design is the first step
Synthesis cannot begin unless there is already a short double- stranded region to be copied
If the matching occurs, then DNA polymerase can bind the dNTPs
PCR Steps
Denaturation
Annealing Extension
Outcome of PCR
Exponential increase in the number of target molecules in successive cycles
After 22 cycles you would expect to have over a million target molecules
2^22 = 4,194,304
How many cycles of PCR are done in practice
25-35 cycles are usually preformed to ensure sufficient amplification of the desired region of the template
Advantages of PCR
PCR is a straightforward technique
Amplify DNA starting with a very small sample (as little as one molecule), you can make large quantities of DNA
It allows the amplification of a specific region of DNA without reliance on pre- existing restriction sites
Limits of PCR
DNA sequence information is needed before you begin
The length of the region that can be amplified:
PCR works well over short stretches of DNA up to about 2 kb
New systems make it possible to amplify target regions of up to 20 kb
DNA Sequencing
Determine the order of the nucleotides that make up a DNA
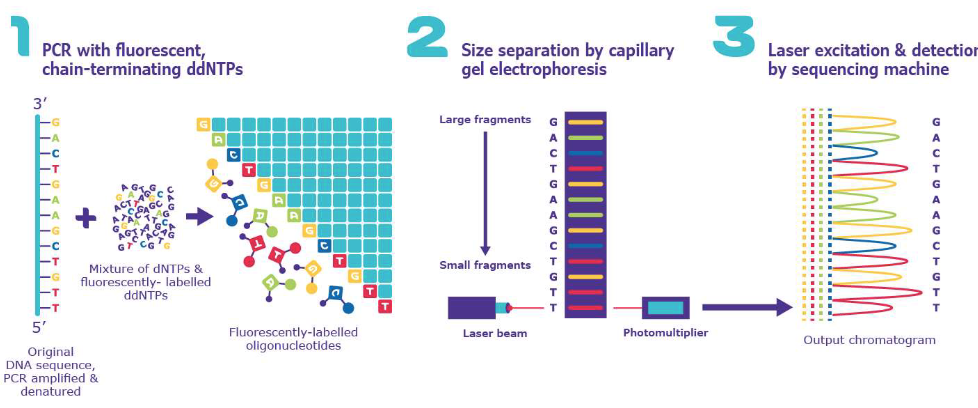
Sanger Sequencing
Most commonly used technique for sequencing DNA
Depends on PCR
Sanger sequencing gives high-quality sequence for stretches of DNA (up to about 900 base pairs)
It's typically used to sequence individual pieces of DNA, such as bacterial plasmids
Sanger sequencing is expensive and inefficient for larger-scale projects, such as the sequencing of an entire genome
Sanger Sequencing Materials
A single-stranded DNA Template
A DNA primer
A DNA polymerase
Deoxynucleoside triphosphates (dNTPs)
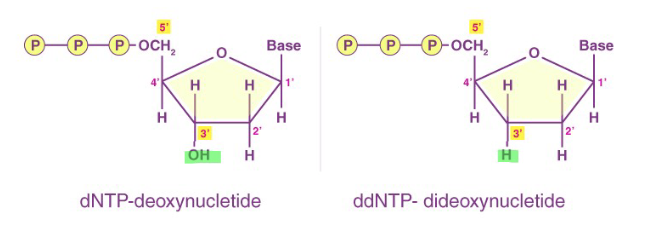
Modified di-deoxynucleotide triphosphates (ddNTPs)
Modified di-deoxynucleotide triphosphates (ddNTPs) (Sanger Sequencing)
The ddNTPs are fluorescently labeled for detection
Each base is labeled differently
Lack the 3'-OH group required for the formation of a phosphodiester bond between two nucleotides
Sanger Sequencing: Steps
Many DNA polymerases attempting to copy many copies of the DNA template strand
Eventually we will have a dideoxy-terminated strand corresponding to every possible nucleotide in the original DNA strand
next-generation sequencing (NGS)
To sequence an entire genome efficiently and cost-effectively, high-throughput sequencing technologies, also known as next-generation sequencing (NGS), are commonly used.
These technologies allow for the simultaneous sequencing of millions of DNA fragments, enabling rapid and comprehensive genome analysis.