Cellular Respiration
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
30 Terms
cellular respiration as a redox reaction
involves transfer of electrons and hydrogen from one substance to another
oxidation reaction
loss of electrons and hydrogen atoms, release energy ex. C6H12O6 → 6CO2
reduction reaction
gain of electrons and hydrogen atoms, gain energy ex. 6O2 → 6H2O
electron carriers
NAD+ (nicotinamide adenine dinucleotide) and FAD (flavine adenine dinucleotide) gradually and over multiple steps oxidize glucose by accepting electrons and hydrogen (becoming reduced) from glucose during cell respiration
role of NAD as a carrier of Hydrogen
reduction of NAD occurs due to NAD accepting atoms of hydrogen
glucose oxidized when hydrogen atoms removed
each hydrogen atom consists of one electron and one proton, NAD+ accepts 2H+ and 2e- and is reduced
NAD+ + 2H+ + 2e- → NADH + H+
role of FAD as a carrier of Hydrogen
FAD → FADH2
FAD reduced
stages of cellular respiration
glycolysis
link reaction
Krebs/citric acid cycle
electron transport chain and chemiosmosis
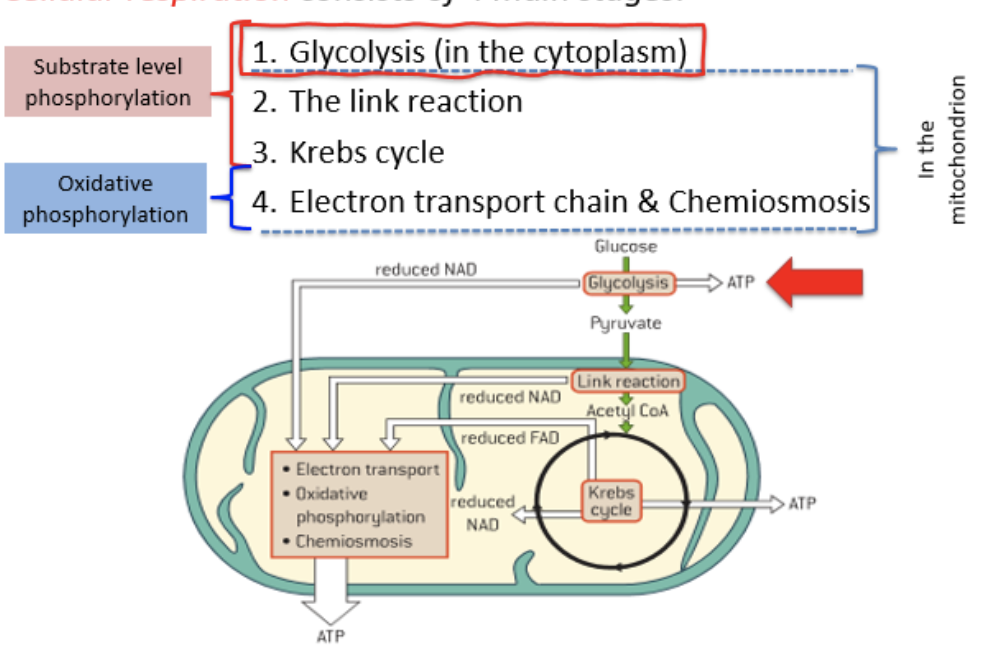
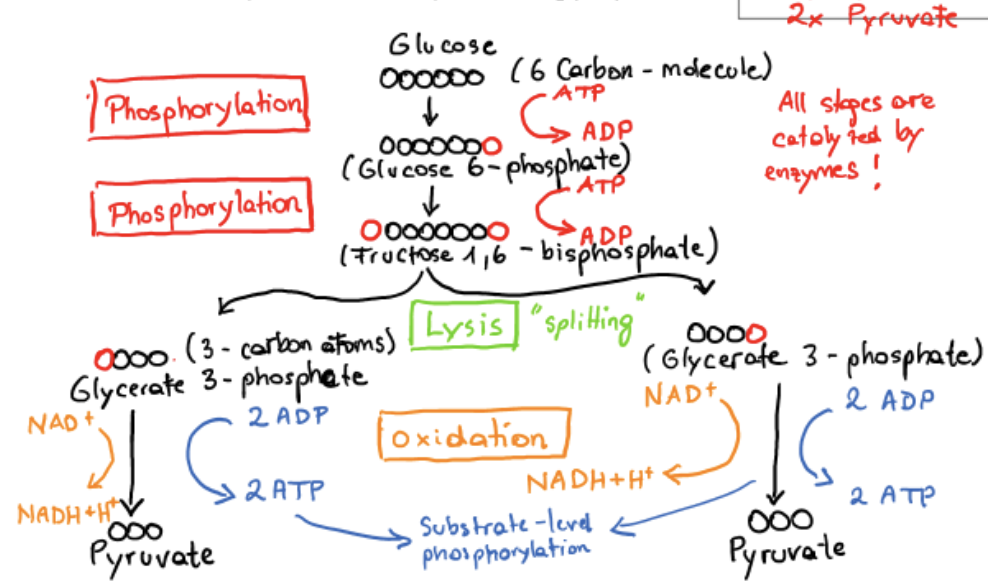
glycolysis
conversion of glucose to pyruvate
preparatory phase
payoff phase
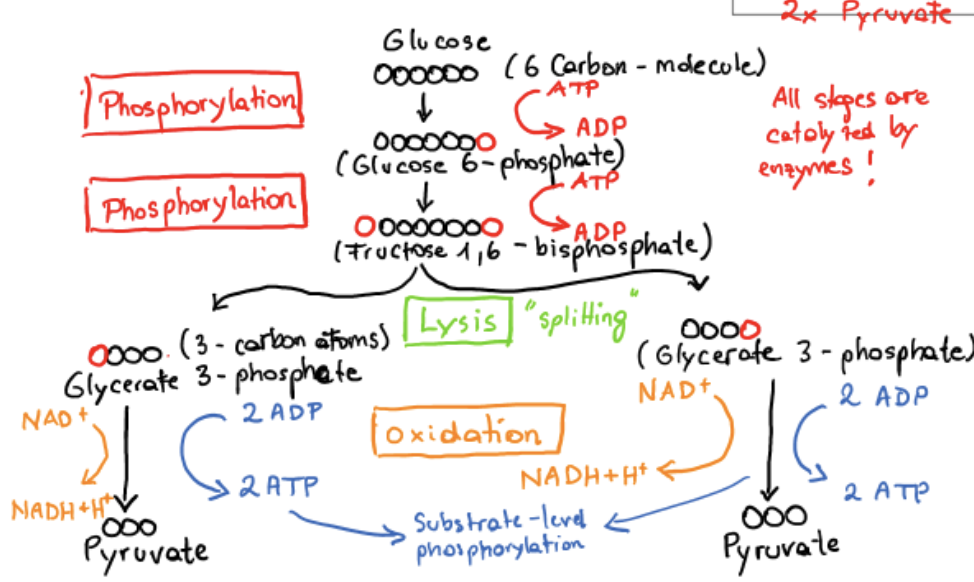
preparatory phase of glycolysis
phosphorylation of glucose, conversion to glyceraldehyde 3-phosphate
makes molecule less stable, lowers activation energy for subsequent splitting to pyruvate
prevents phosphorylated molecule from being transported through the cell membrane (not recognized by protein pumps)
energy released in hydrolysis of ATP used for attachment of PO43-
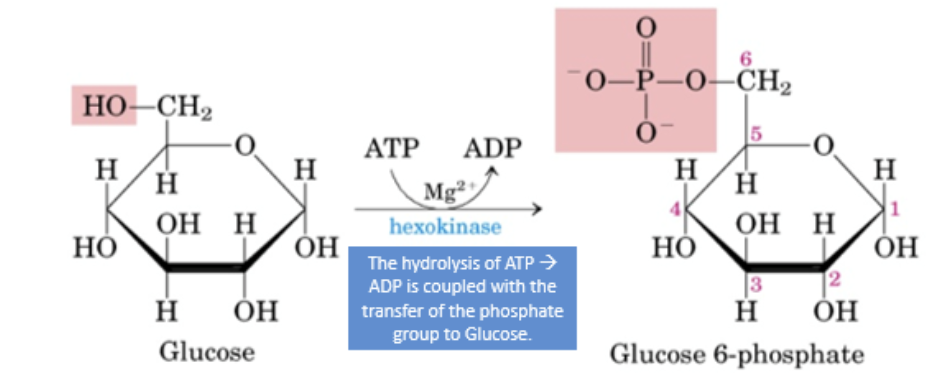
phosphorylation
addition of a PO43- group
payoff phase of glycolysis
oxidative conversion of glyceraldehyde 3-phosphate to pyruvate and the coupled formation of ATP and NADH, net production of 2 ATP
uses of pyruvate
anaerobic respiration (alcoholic fermentation, lactic acid fermentation) and aerobic respiration depending on oxygen availability
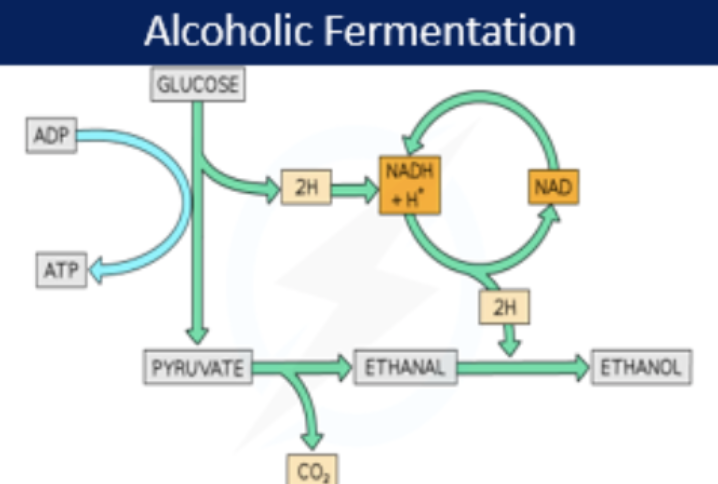
anaerobic respiration
NADH + H+ donates its H+ and e- to pyruvate during ethanol or lactate production, respectively
NAD+ becomes available again so that e- and H+ can continue to be transferred from glucose to NAD+ when oxidized
thus pyruvate synthesis during glycolysis continues
alcoholic fermentation
C6H12O6 → 2 pyruvate → 2 Ethanol + 2CO2 (net 2 ATP)
in yeast (eukaryotic cell) and some bacteria (prokaryotic cell)
cytoplasm
yeast as an example of anaerobic cell respiration
facultative anaerobe single-celled fungus, can respire aerobically or anaerobically
breaks down starch and sugars in dough by alcoholic fermentation to make CO2 and ethanol
CO2 released in fermentation trapped in dough, causing bread to rise
bread baked in oven to kill yeast and trap ethanol
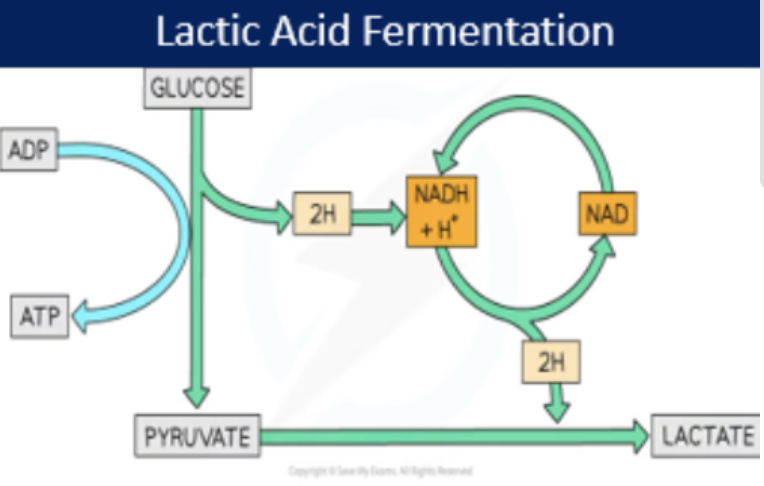
lactic acid fermentation
C6H12O6 → 2 Lactate (net 2 ATP)
some bacteria (prokaryotic cell) and some mammals
cytoplasm
aerobic respiration
C6H12O6 → 6CO2 + 6H2O (net 32-34 ATP)
before the link reaction
pyruvate/pyruvic acid enters mitochondrial matrix by facilitated diffusion to be processed further
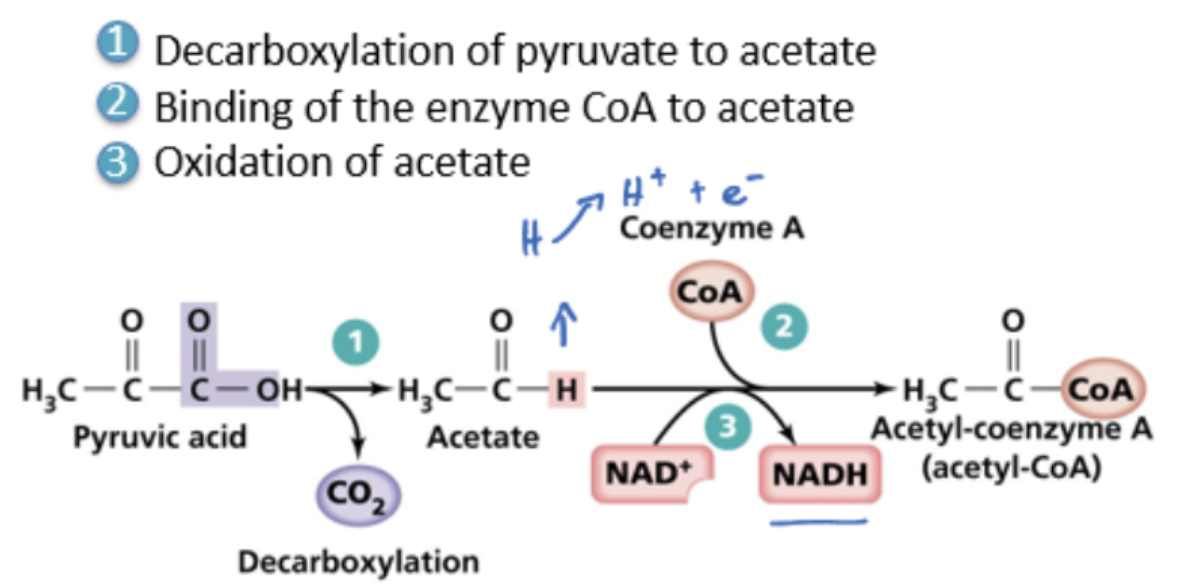
link reaction
decarboxylation of pyruvate to acetate
CO2 leaves cell, diffuses into the bloodstream and is expired
binding of enzyme CoA to acetate
oxidation of acetate into acetyl-coenzyme A
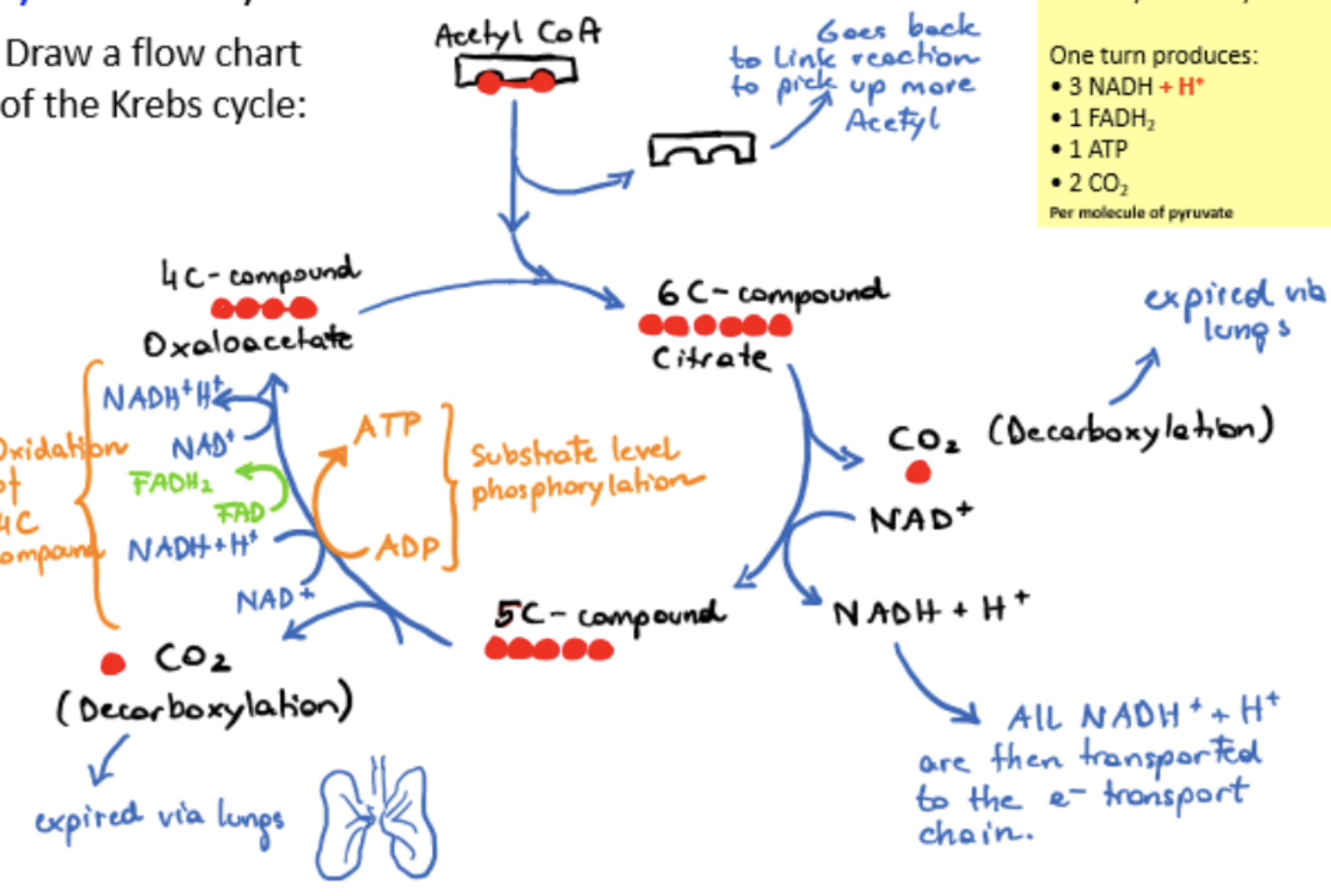
Krebs/citric acid cycle
oxidation and decarboxylation of acetyl groups
acetyl groups (2C) fed into cycle by transfer from coenzme A to oxaloacetate (4C) → citrate (6C)
citrate converted back to oxaloacetate by enzyme-catalyzed reactions, two carbons lost through decarboxylation reactions, producing waste product CO2 - each carboxylation reaction paired with reduction of NAD+
4 oxidation reactions release energy, mainly held in electrons removed from oxidation and transferred through reduction of NAD+ and FAD
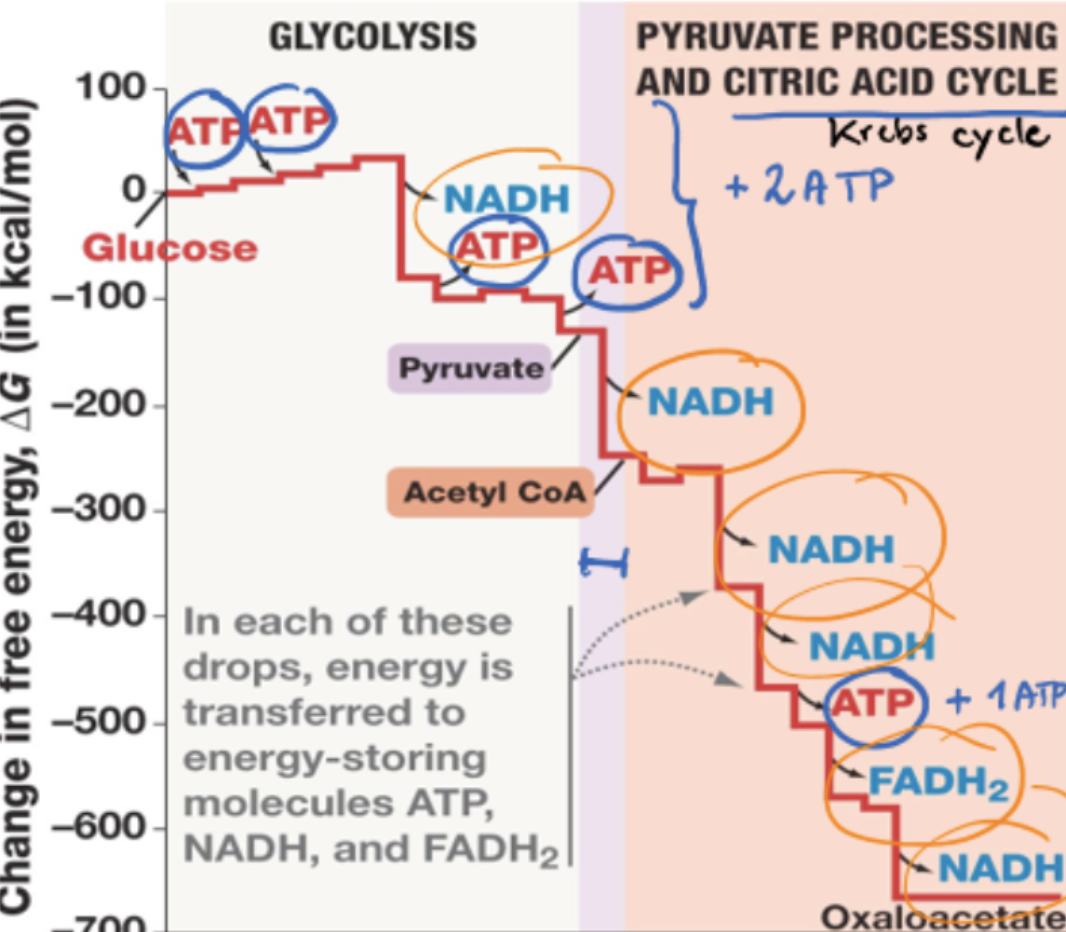
change in free energy graph in electron transport chain
in each drop, energy is transferred to energy-storing molecules NAD+ and FAD, which later become oxidized again in electron transport chain
energy gained from oxidation reaction used to make ATP
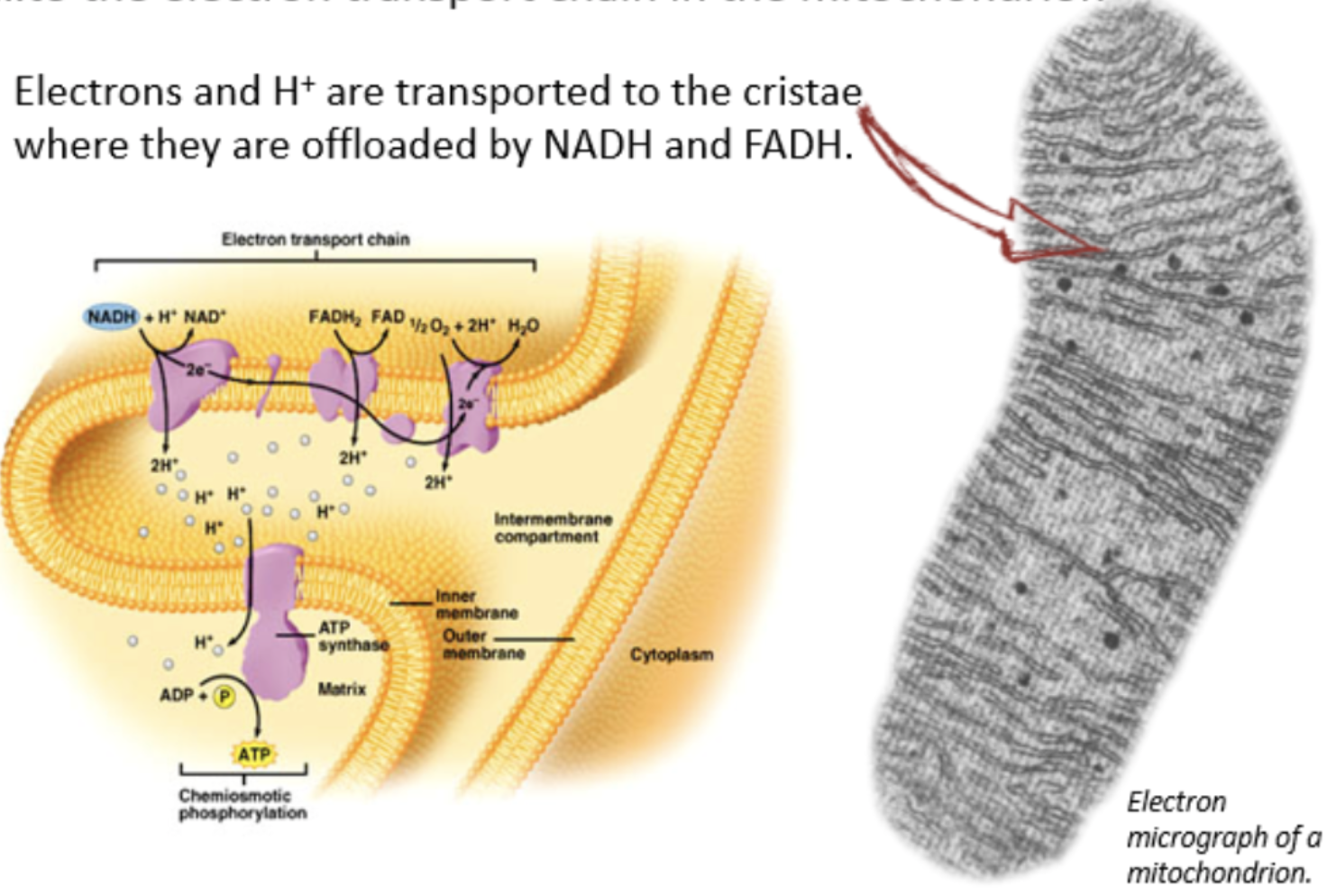
transfer of energy to the electron transport chain
electron carriers NAD+ and FAD bring electrons and hydrogen ions to electron transport chain in cristae of the mitochondria
electron transport chain
reduced electron carriers NADH + H+ and FADH2 from glycolysis and Krebs cycle move to inner mitochondrial membrane
membrane proteins accept electrons from NADH and FADH2
each carrier in chain has slightly higher electronegativity and therefore a stronger attraction for electrons than previous carrier
electrons passed down an energy gradient until they reach the end of the chain
electrons “fall” from higher levels to lower ones, energy released used to pump protons from matrix into intermembrane space against the concentration gradient
proton gradient drives chemiosmosis
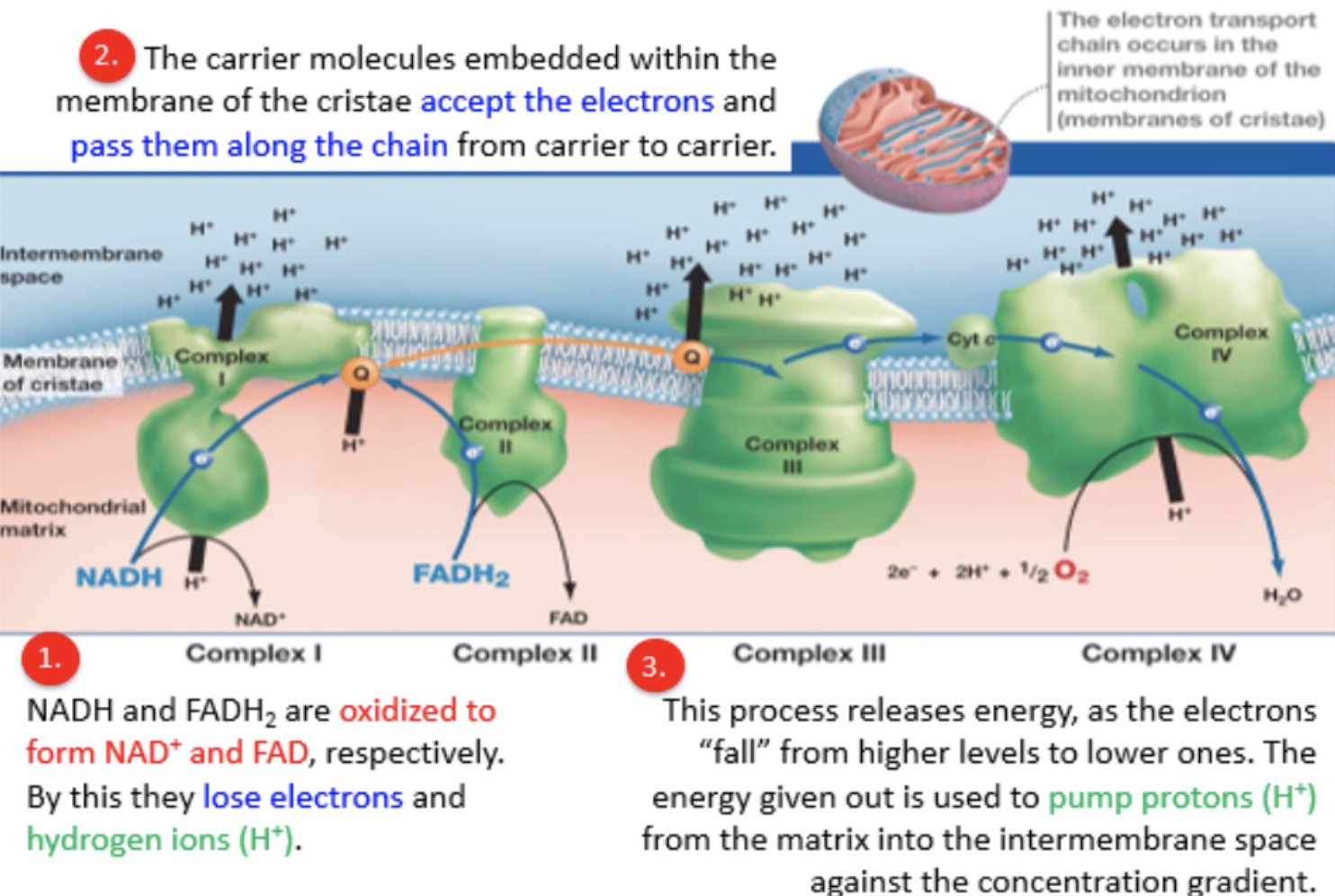
membrane proteins in electron transport chain
integral carrier and channel proteins embedded within phospholipid bilayer have a high tendency to become reduced by accepting electron
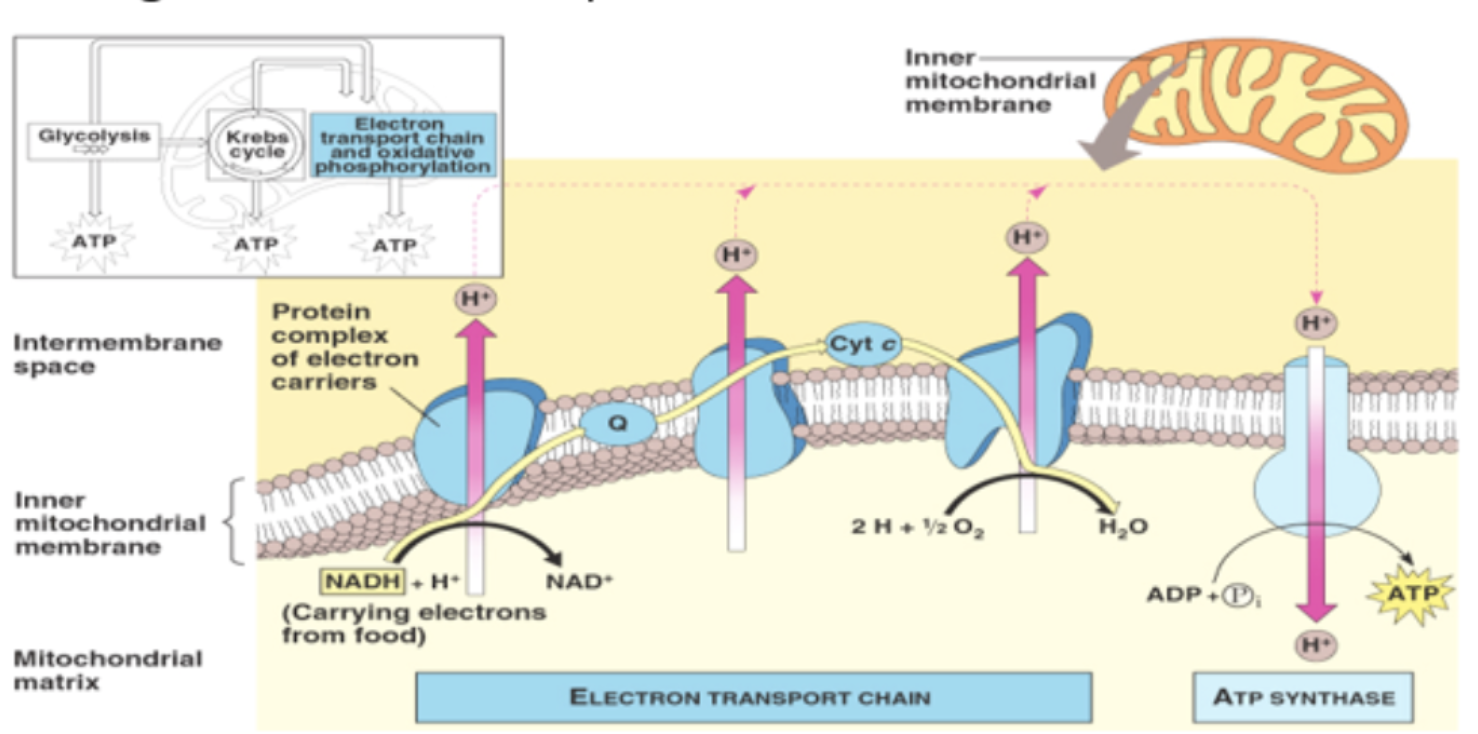
chemiosmosis
transfer of H+ sets up concentration gradient across the membrane as H+ accumulates in the intermembrane space
protons follow natural concentration gradient by moving through the ATP synthase
oxidative phosphorylation
oxygen as the terminal electron acceptor
oxidative phosphorylation in between chemiosmosis and ATP synthesis
movement of protons through ATP synthase releases energy used to phosphorylate ADP to ATP
ATP synthase
complex of integral proteins located in the mitochondrial inner membrane where it catalyzes the synthesis of ATP from ADP and phosphate, driven by a flow of protons
role of oxygen as terminal electron acceptor
in the reduction of the oxygen molecule, the O2 accepts electrons and forms a covalent bond with hydrogen to produce H2O
in the matrix, H+ combines with ½ O2 + 2e- to form water
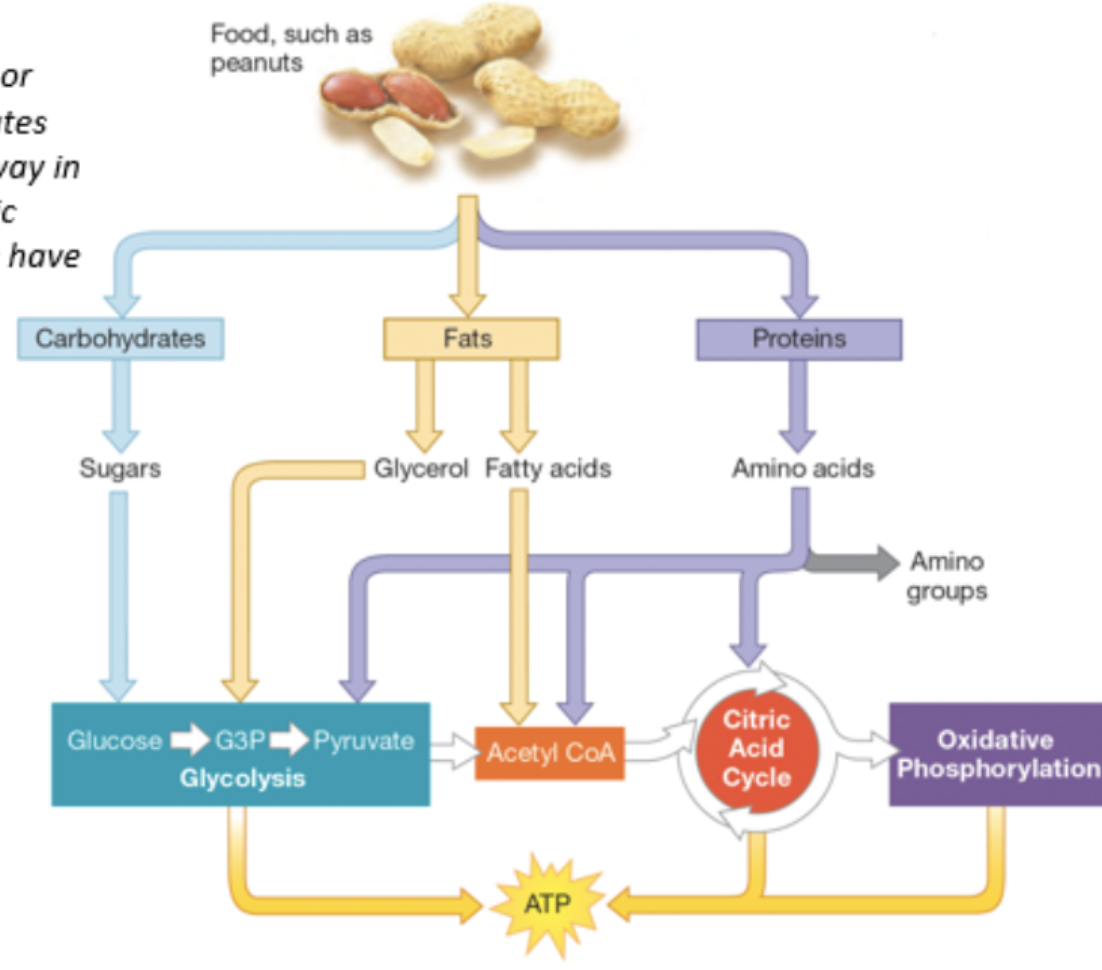
carbohydrates as respiratory substances
simple sugars (glucose or fructose) can be used straight away in glycolysis and anaerobic respiration
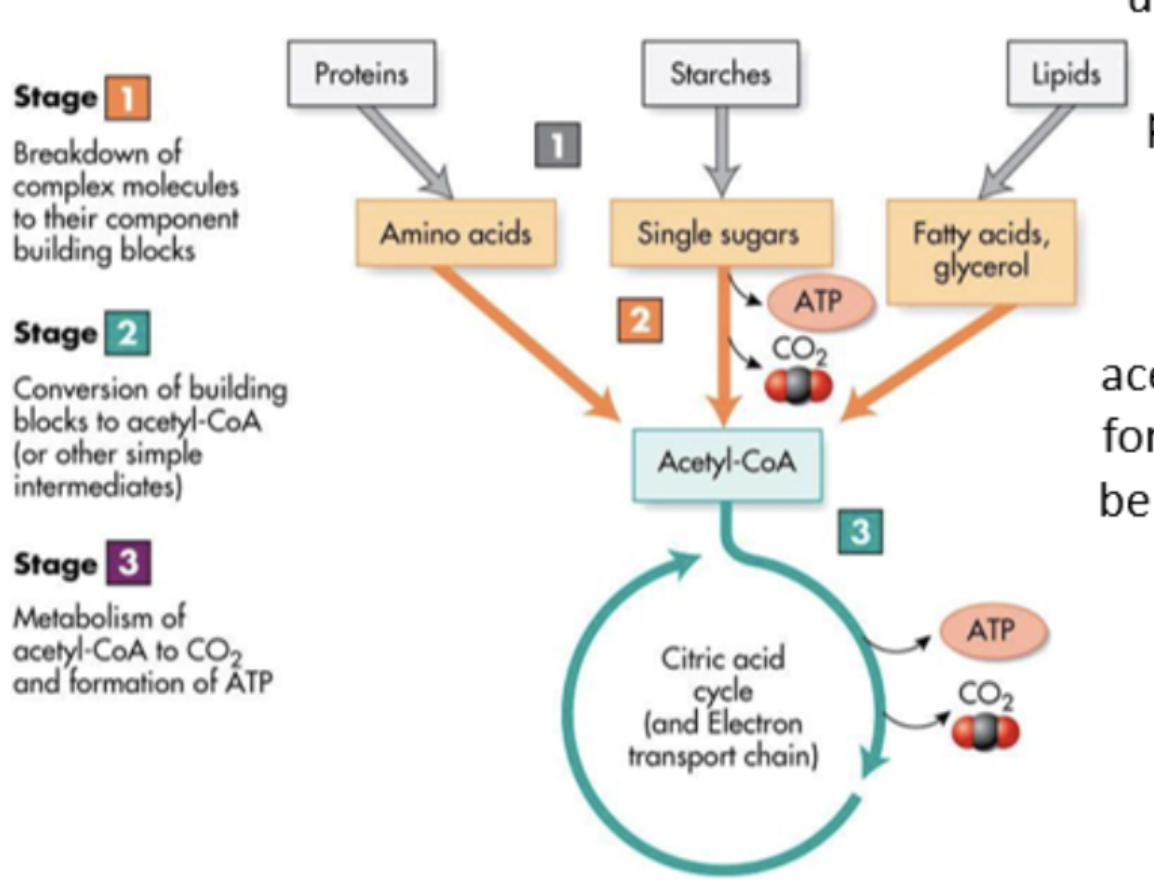
lipids as respiratory substances
lipids broken down into glycerol and fatty acids, fatty acids converted to acetyl groups to be used in Krebs cycle