ORGANIC COMPOUNDS
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
16 Terms
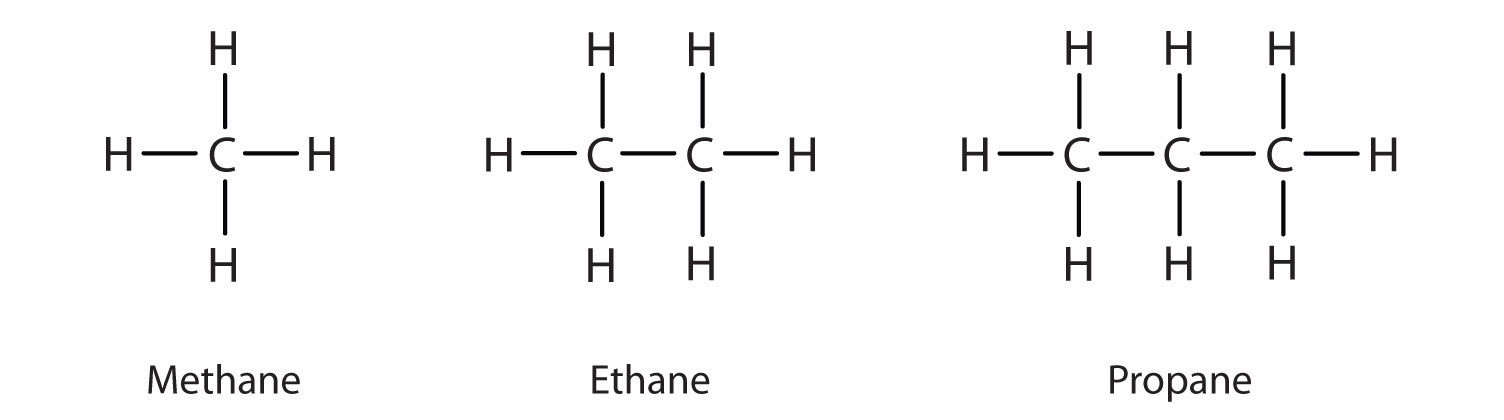
alkanes
hydro carbons, only C-C and C-H SINGLE bond
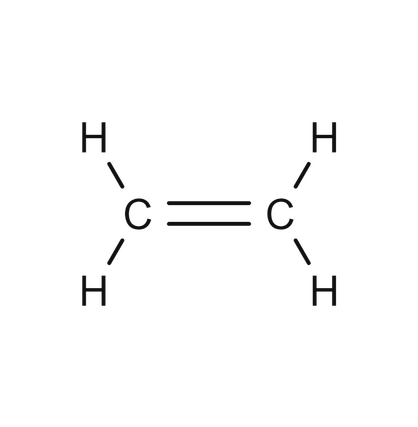
alkenes
hydro carbons, at least one C-C and C-H DOUBLE bond
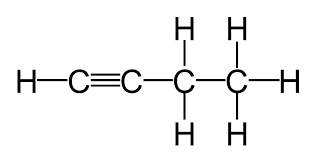
alkynes
hydro carbons, at least one C-C and C-H TRIPLE bond
alcohol
an OH (hydroxyl group) attached to a saturated Carbon atom
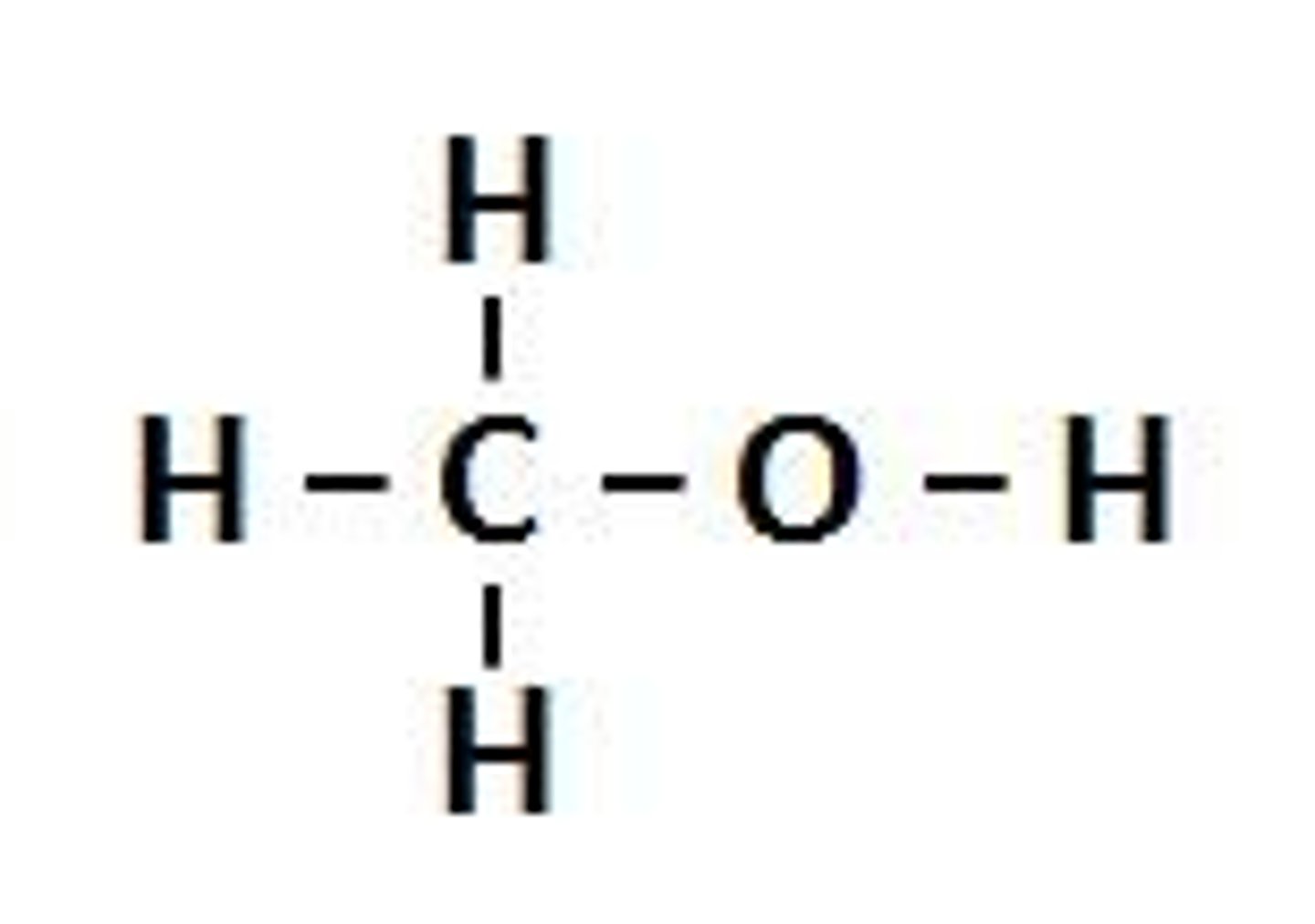
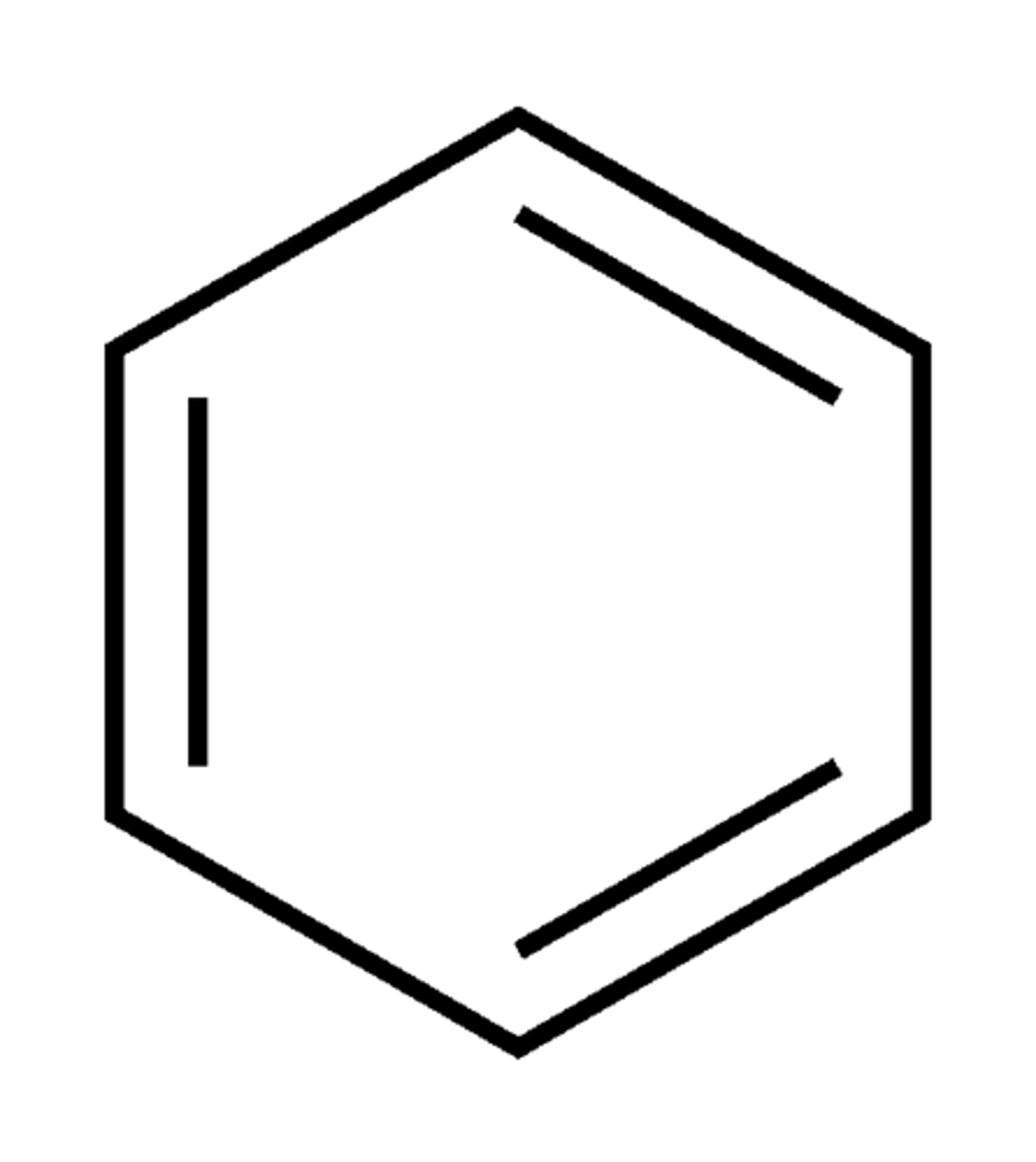
Benzene
aromatic compound (unsaturated Carbond bonds) alkenes
Chemical formula: C6H12
ether
oxygen atom with single bonds to 2 C groups
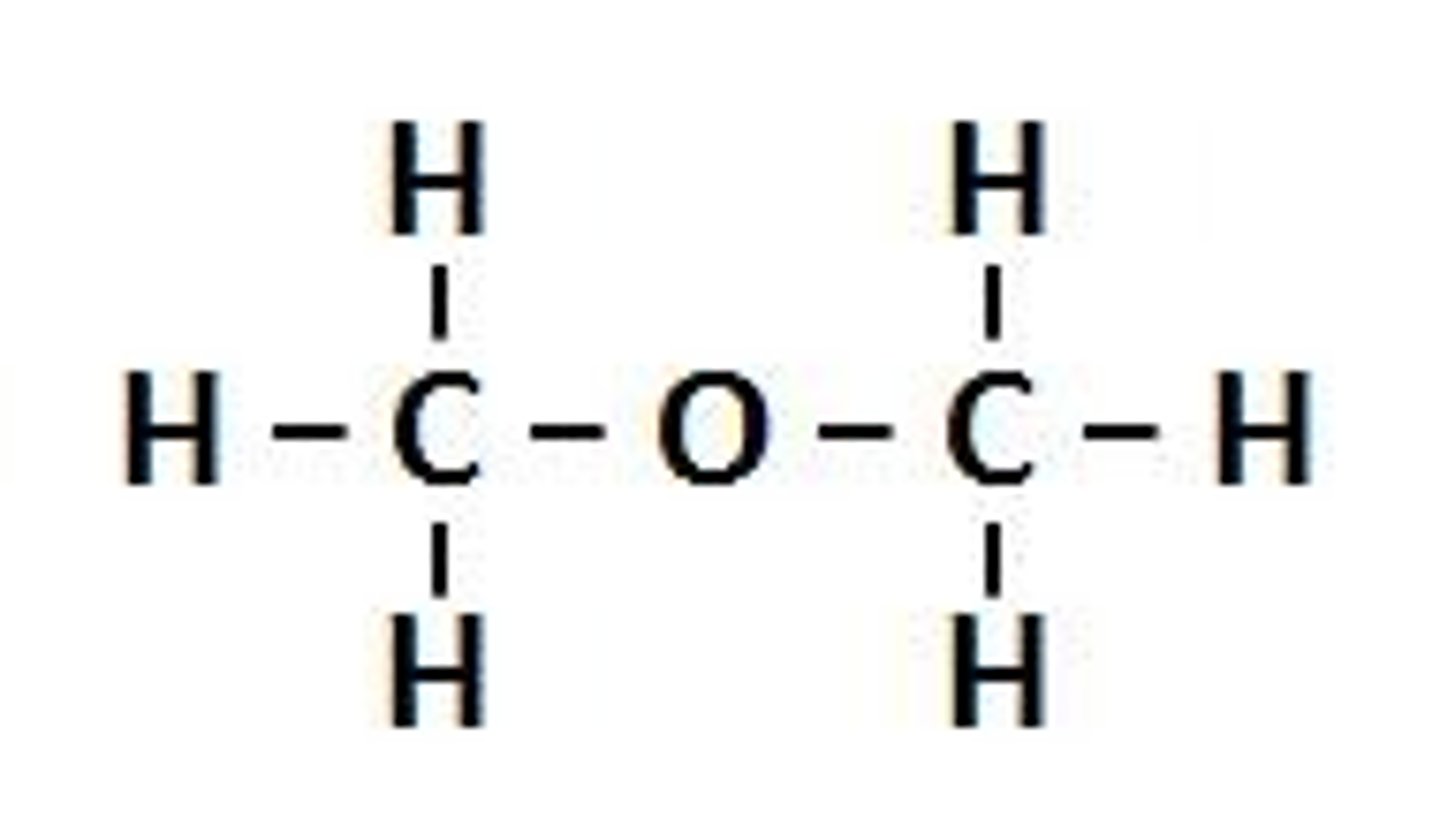
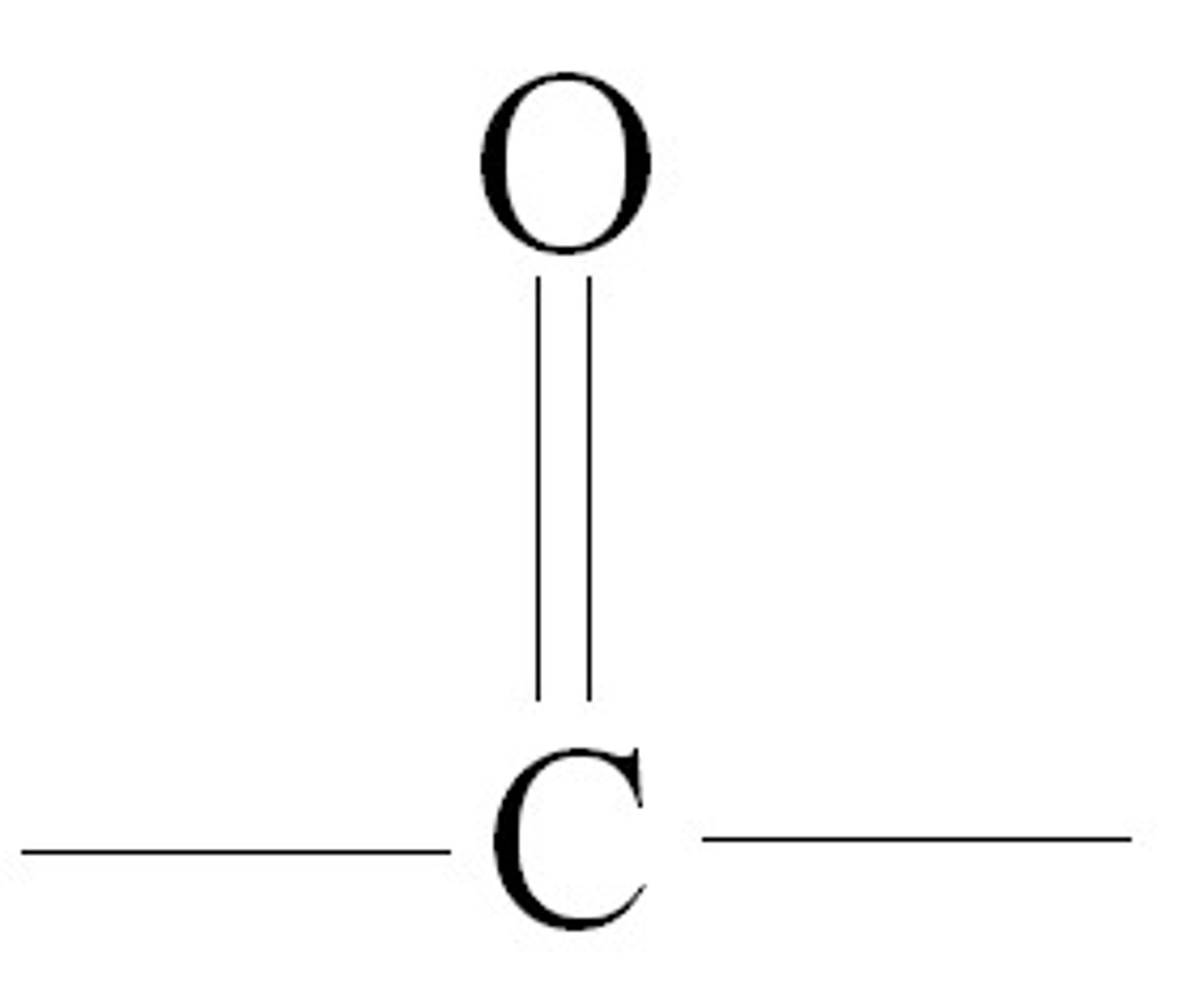
carbonyl
a Carbon with double bonds with an Oxygen
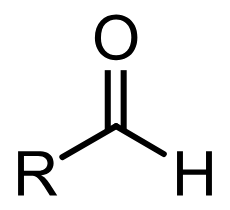
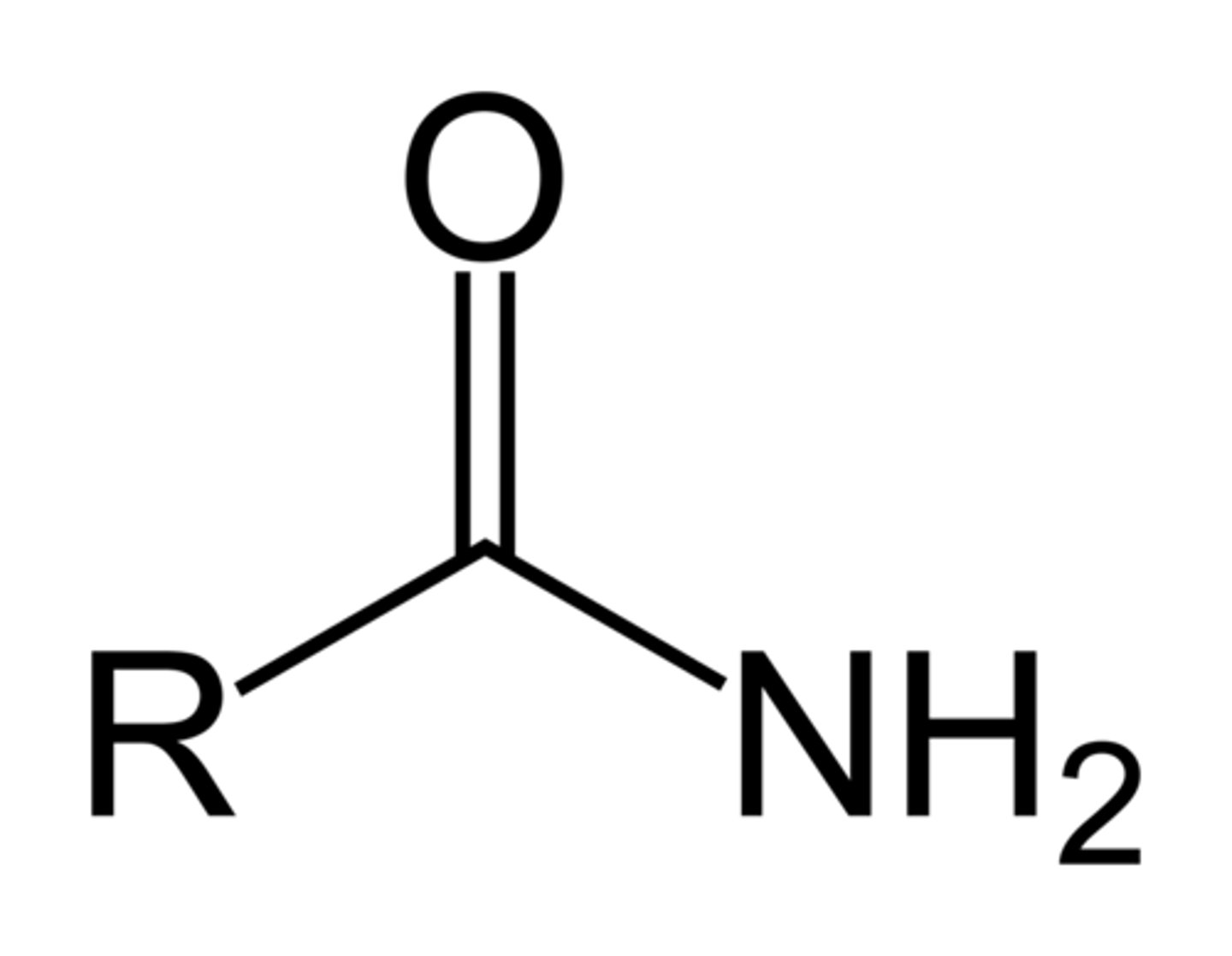
aldehyde
carbonyl group bonded to R and H
ketone
carbonyl group bonded to two R groups
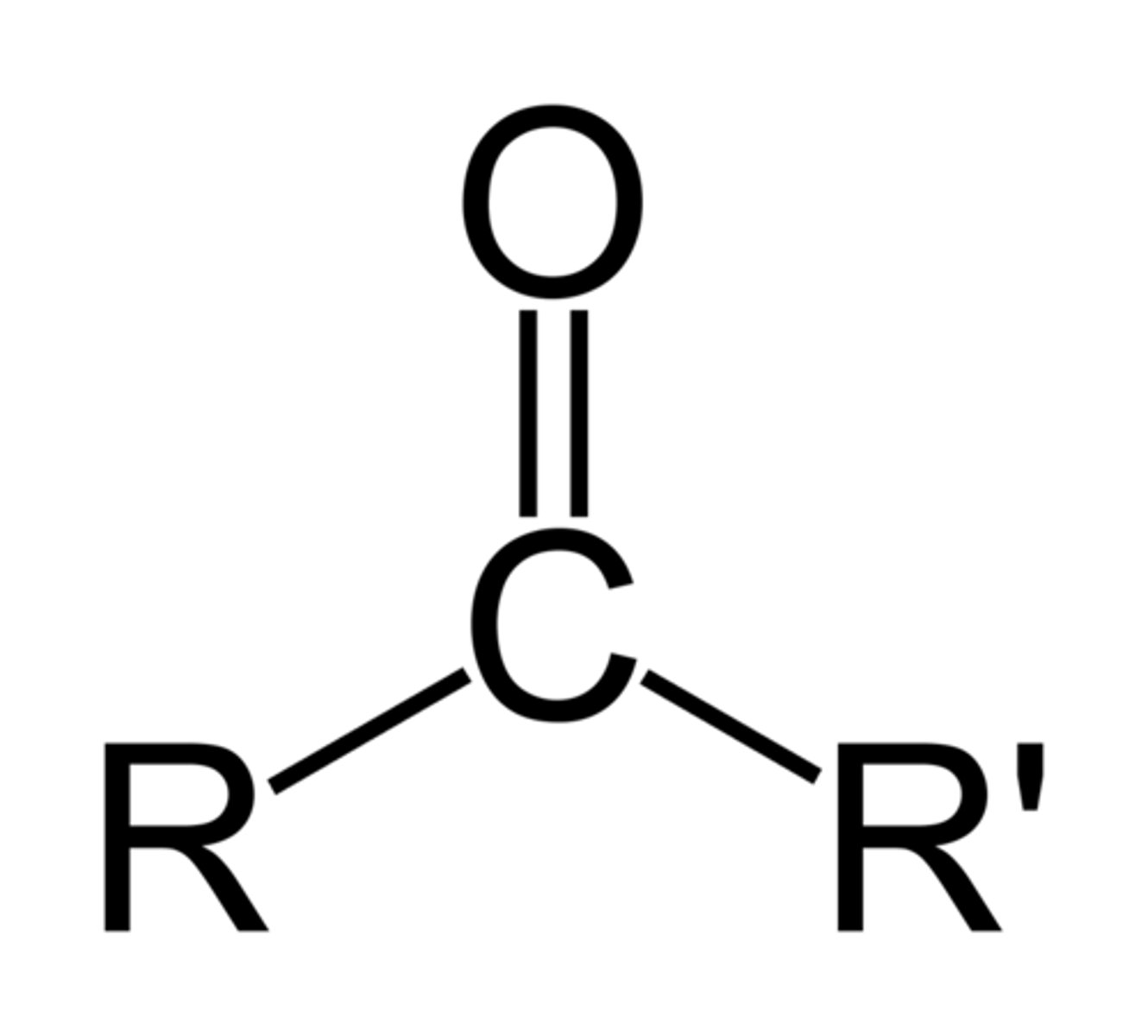
carboxylic acid
carbonyl group bonded to R and hydroxyl (OH)
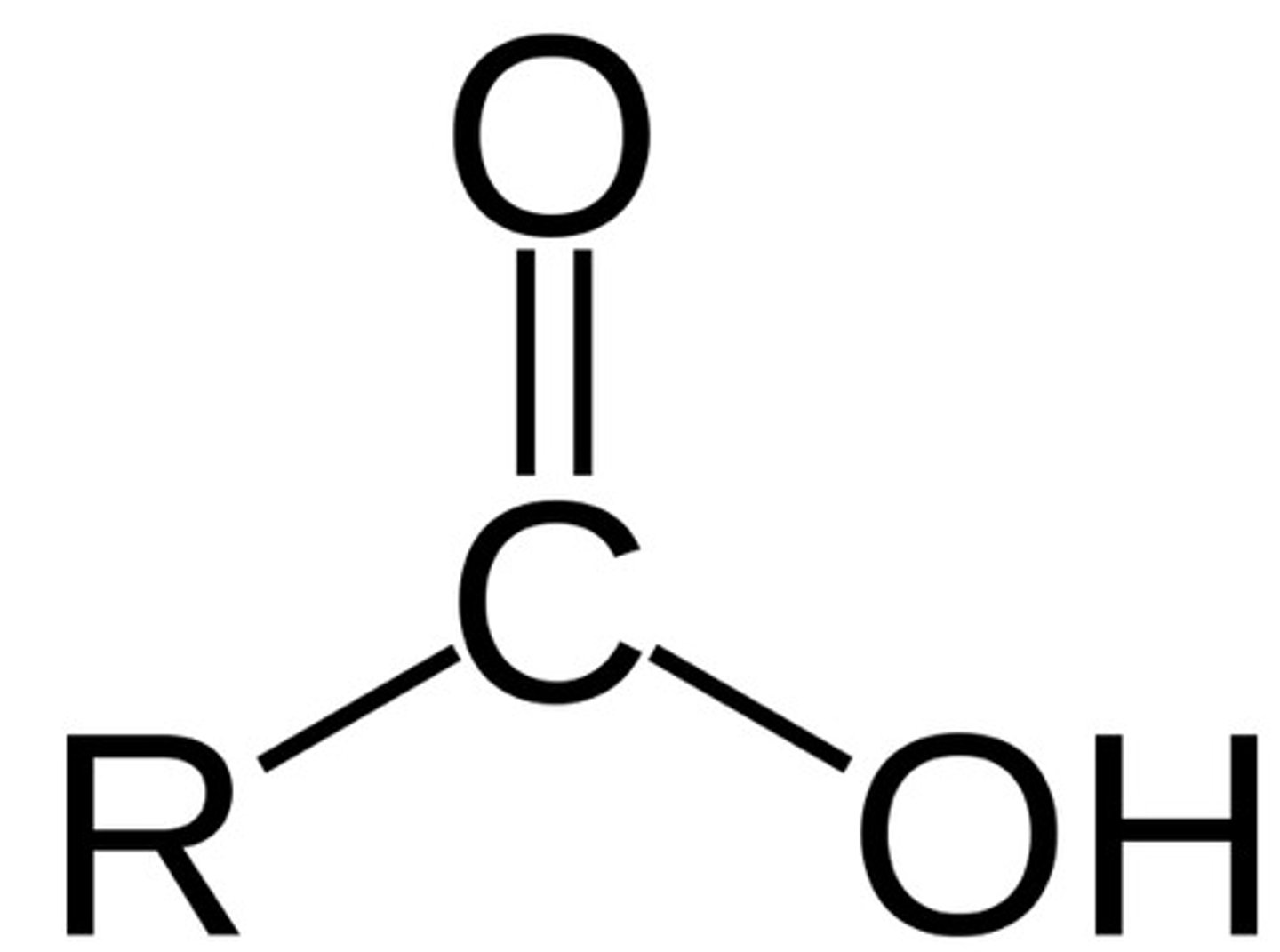
Ester
carbonyl group bonded to R and OR group
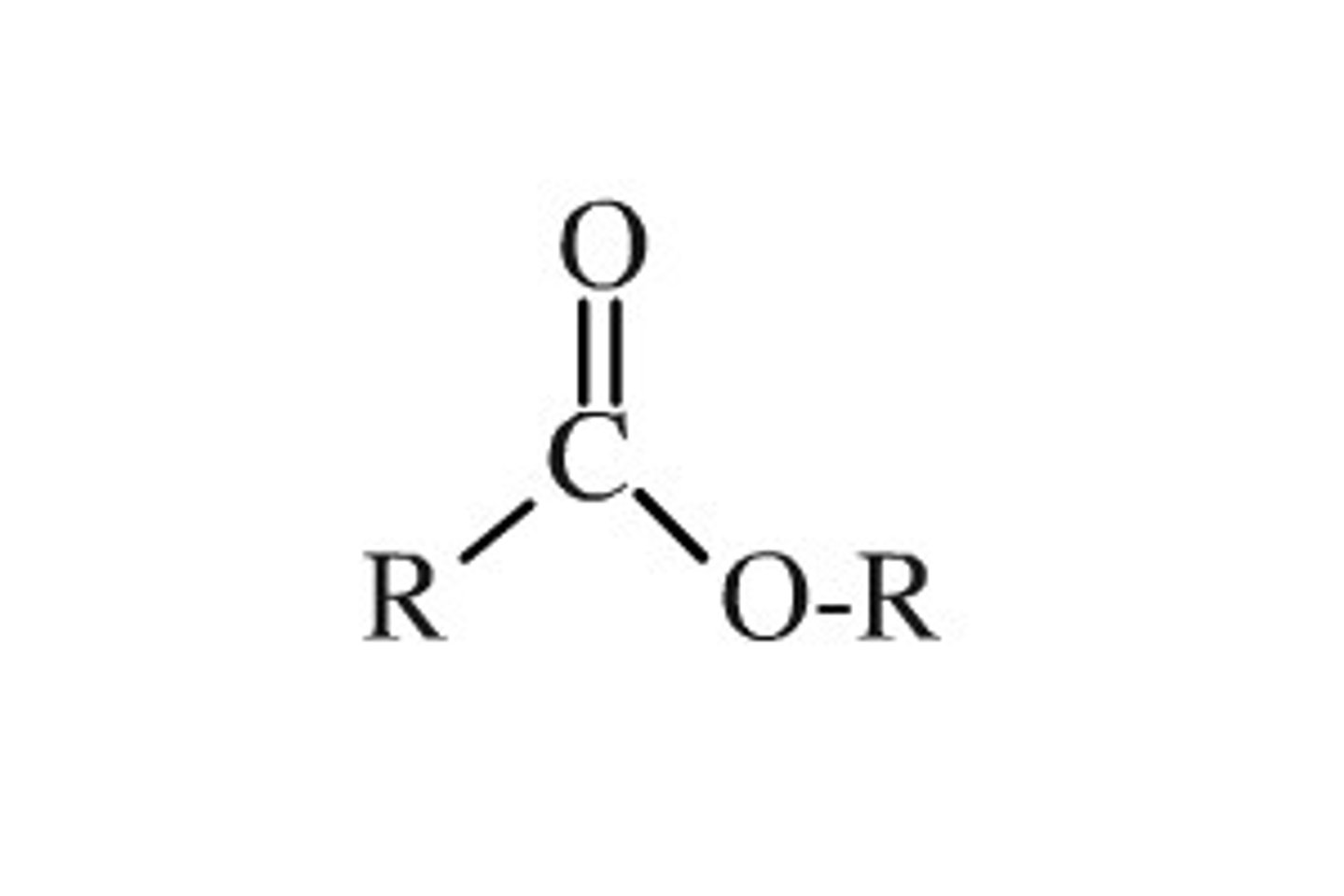
amide
carbonyl group bonded to R and N group
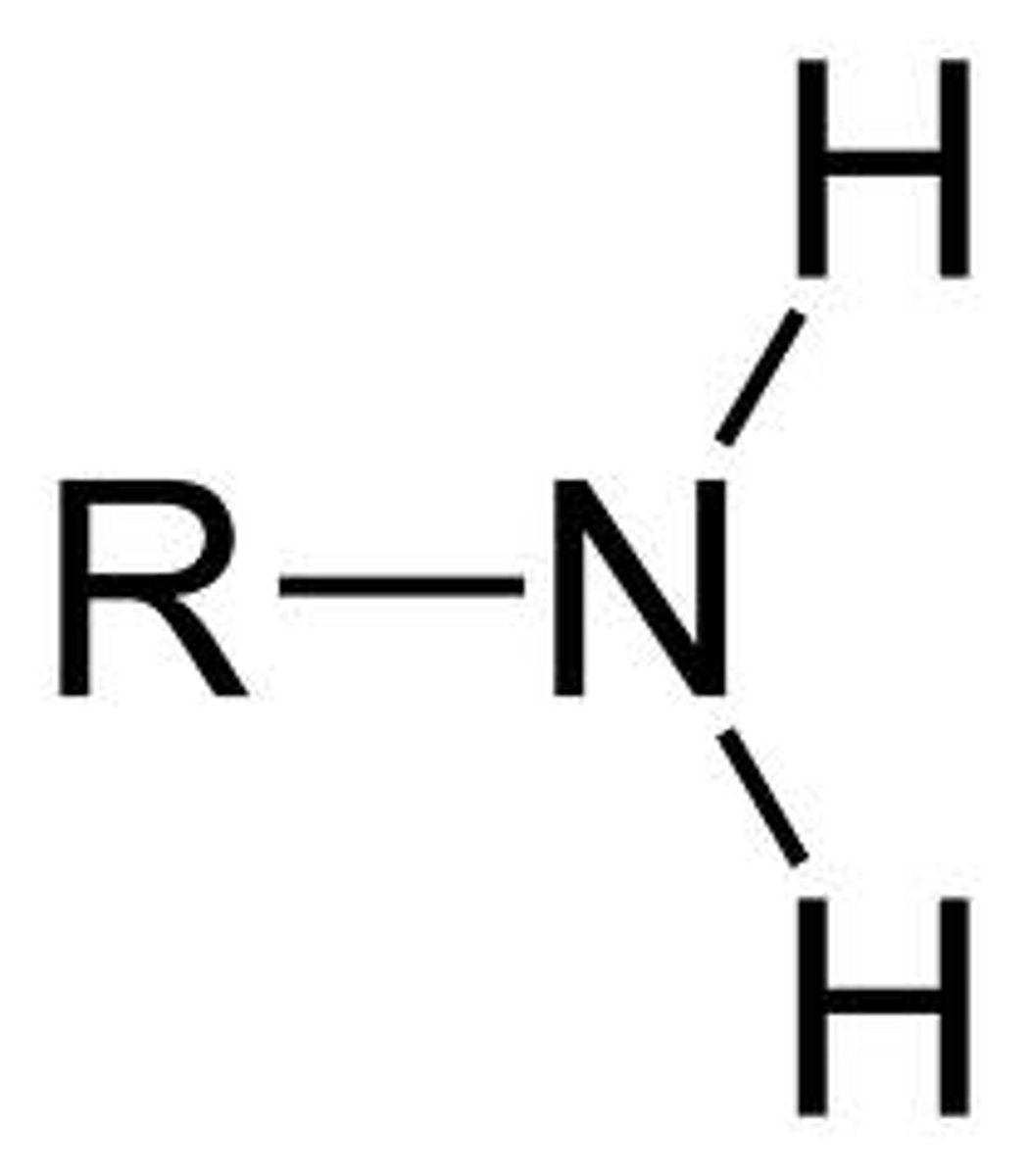
amine
N group bonded to R groups, NO CARBON
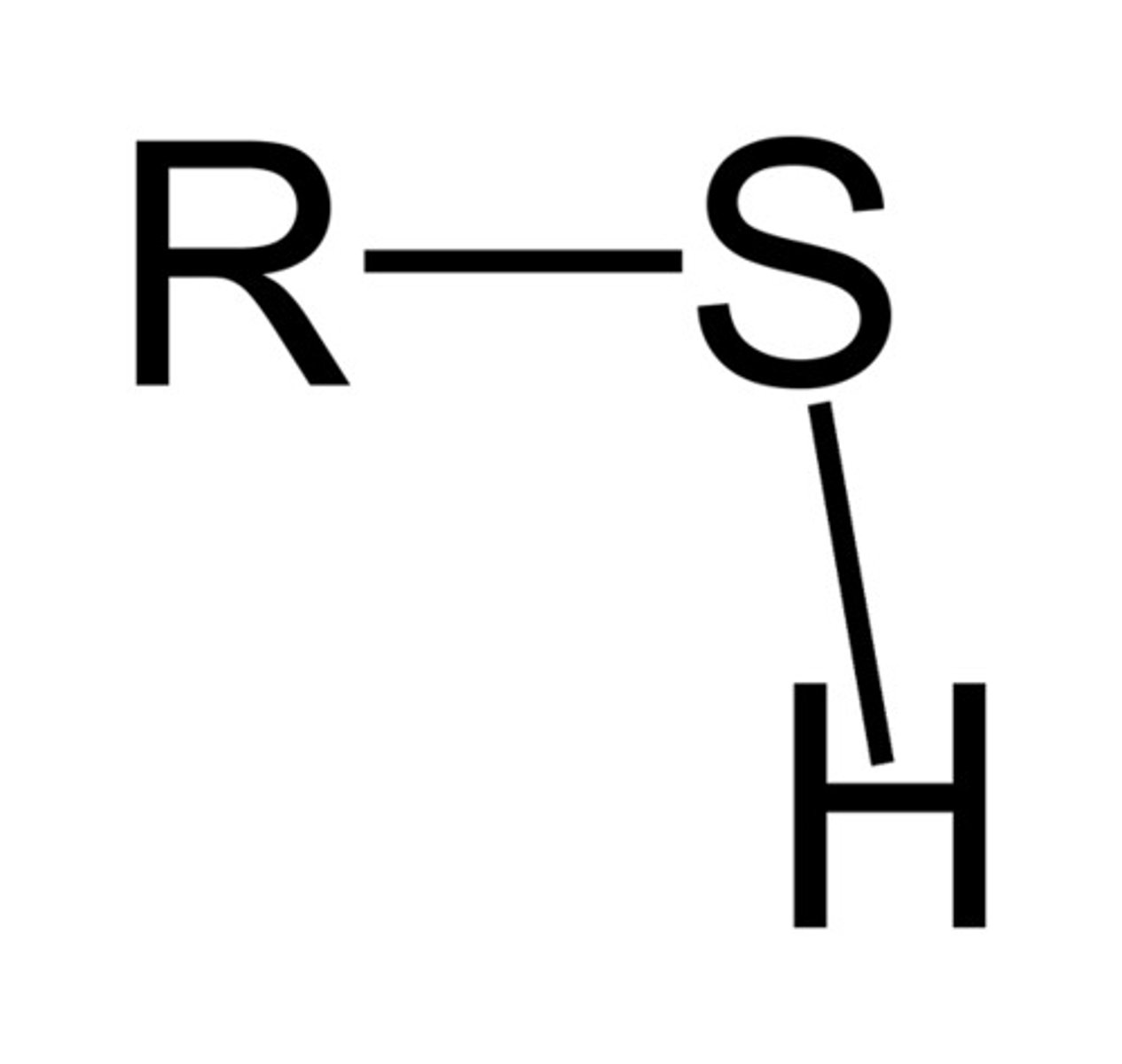
thiol
S group bonded to R and H group
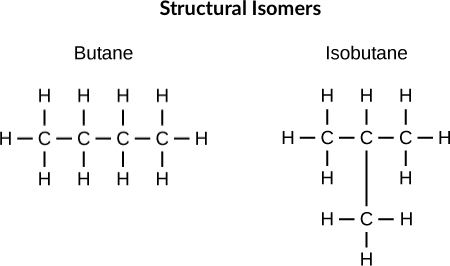
structural isomers
same formula, different compound
conformational isomers
same compound and formula, rotated