MCAT Chemistry - Equilibrium
1/13
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
14 Terms
chemical equilibrium
the state in which both the reactants and products are present in concentrations which have no further tendency to change with time, so that there is no observable change in the properties of the system; V
irreversible reactions
the reaction proceeds in one direction only, the reaction goes to completion, and the maximum amount of product formed is determined by the amount of limiting reagent initially present
Reversible reactions
the reaction can proceed in one of two ways: forward (toward the products or “to the right”) and reverse (toward the reactants or “to the left”); usually do not proceed to completion because the products can react together to reform the reactants
dynamic equilibrium
which the rate of the forward reaction equals the rate of the reverse reaction and the concentrations of the products and reactants remain constant
static equilibrium
reaction stop
entropy
the measure of the distribution of energy throughout a system or between a system and its environment; a reaction will reach equilibrium when the system’s entropy is at a maximum and the Gibbs free energy of the system is at a minimum.
law of mass action
if the system is at equilibrium at a constant temperature, then the following ratio is constant
The concentrations of pure solids and pure liquids do not appear in the equilibrium constant expression. This is because the equilibrium expression is technically based on the activities of compounds, not concentrations; the activities of pure solids and liquids are defined to be 1. For the purposes of the MCAT, there is a negligible difference between concentration and activity.
Keq is characteristic of a particular reaction at a given temperature; the
equilibrium constant is temperature-dependent.
The larger the value of Keq, the farther to the right the equilibrium position.
If the equilibrium constant for a reaction written in one direction is Keq, the equilibrium constant for the reverse reaction is 1/Keq.
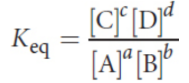
reaction quotient (Q)
At any point in time during a reaction, we can measure the concentrations of all of the reactants and products to indicate how far the reaction has proceeded toward equilibrium
Q < Keq
the forward reaction has not yet reached equilibrium.
There is a greater concentration of reactants (and smaller concentration of products) than at equilibrium.
The forward rate of reaction is increased to restore equilibrium.
Q = Keq
the reaction is in dynamic equilibrium.
The reactants and products are present in equilibrium proportions.
The forward and reverse rates of reaction are equal.
Q > Keq
the forward reaction has exceeded equilibrium.
There is a greater concentration of products (and smaller concentration of reactants) than at equilibrium.
The reverse rate of reaction is increased to restore equilibrium.
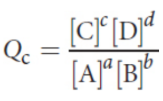
Le Châtelier’s principle
if a stress is applied to a system, the system shifts to relieve that applied stress
Changes in Concentration
the system will always react in the direction away from the added species or toward the removed species
Changes in Pressure and Volume
only chemical reactions that involve at least one gaseous species will be affected by changes in the system’s pressure and volume; The system will move toward whichever side has the lower total number of moles of gas
Changes in Temperature
If a reaction is endothermic (ΔH > 0), heat functions as a reactant; if a reaction is exothermic (ΔH < 0), heat functions as a product.
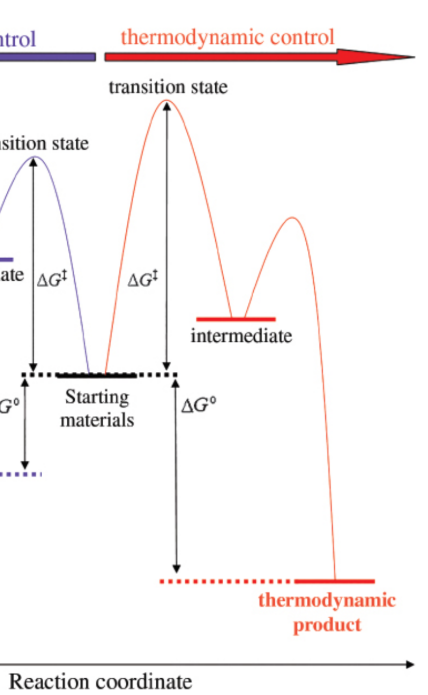
kinetic product
products at lower temperatures; form faster than the thermodynamic products
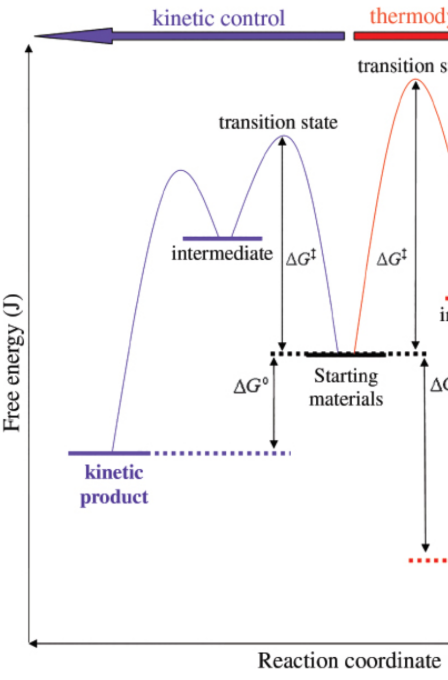
thermodynamic product
products at higher temperatures; form slower than the kinetic products