Enzymes and Activation Energy: Biological Reaction Catalysts
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
17 Terms
What role do enzymes play in chemical reactions?
Enzymes help chemical reactions take place at lower energy levels, allowing the body to use less energy.
Do enzymes supply energy for reactions?
No, enzymes do not supply energy for reactions.
What are enzymes primarily composed of?
Enzymes are almost always proteins.
Where are enzymes typically written in a chemical reaction?
Enzymes are usually written above the reaction arrow.
What is activation energy?
Activation energy is the amount of energy required to start a reaction.
What is the transition state in a chemical reaction?
The transition state is a temporary state between reactants and products, occurring before new bonds are formed.
What provides activation energy for reactions?
Activation energy can be provided by thermal energy or catalysts like enzymes.
What is an exothermic reaction?
An exothermic reaction is one where energy is released, resulting in products that have less energy than the reactants.
What is an endothermic reaction?
An endothermic reaction is one where energy is absorbed, resulting in products that have more energy than the reactants.
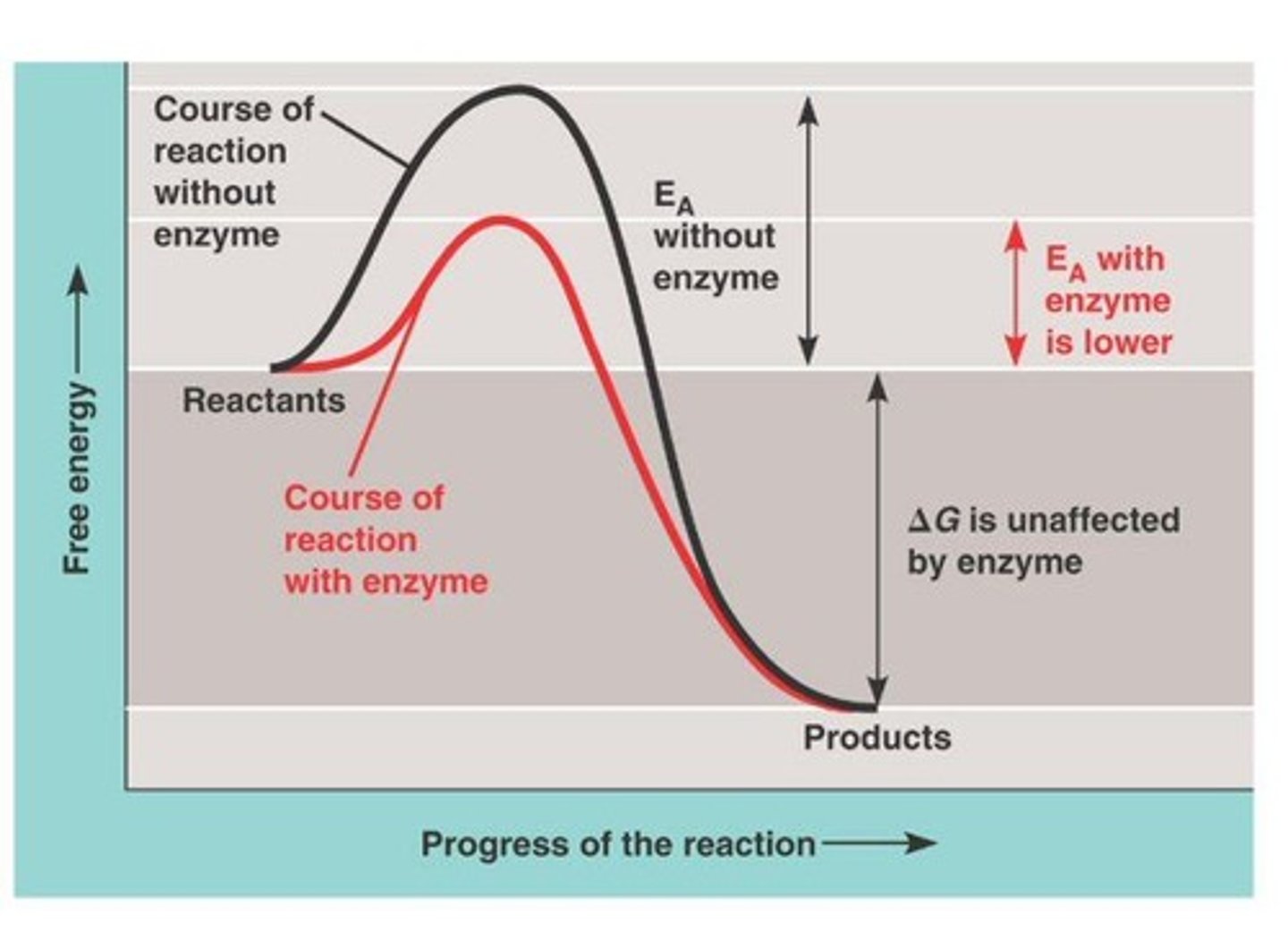
How do enzymes lower activation energy?
Enzymes lower activation energy by bringing reactants together, orienting molecules, and changing the shape of the substrate.
How do charged enzymes assist in reactions?
Charged functional groups in enzymes help attract reactants and break bonds.
What happens to the shape of an enzyme during a reaction?
The enzyme changes shape to alter the shape of the substrate and facilitate bond breaking.
What is the effect of thermal energy on reactions?
Thermal energy can cause more reactions to take place as more energy is released, but it is hard to regulate.
What is the significance of the amount of energy released during a reaction?
The amount of energy released indicates whether the reaction is exothermic or endothermic.
What is the relationship between reactants and products in an exothermic reaction?
In an exothermic reaction, the products have less energy than the reactants.
What is the relationship between reactants and products in an endothermic reaction?
In an endothermic reaction, the products have more energy than the reactants.
What is the role of enzymes as catalysts?
Enzymes act as catalysts by allowing reactions to occur using less energy and can be regulated to control reaction rates.