Arenes, and its preparation
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
26 Terms
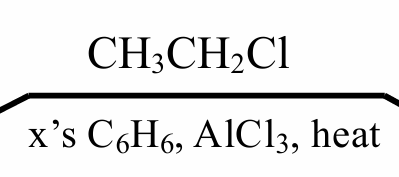
This reagents are used for preparing alkylbenzene by:
Direct alkylation
are hydrocarbons which contain both aliphatic and aromatic units
Arenes
Arenes made up of aromatic and alkane units
alkylbenzenes
Arenes made up of aromatic and alkene units
alkenylbenzenes
Arenes made up of aromatic and alkyne units
alkynylbenzenes
True or False:
the special name of the simplest alkylbenzene is styrene.
FALSE.
toluene-alkyl; styrene-alkenyl
The special name given for the dialkylbenzenes
xylene (o-,p-,m-)
Compounds containing longer side chains are named by:
substituent - alkyl group
parent - benzene
Compounds containing very complicated side chains are named as
phenylalkanes
Compounds containing more than one phenyl group are named as
derivatives of alkanes
True or False:
The alkenylbenzenes are generally named as substituted alkenes, occasionally as substituted benzenes.
TRUE
They are named as substituted alkynes
alkynylbenzenes
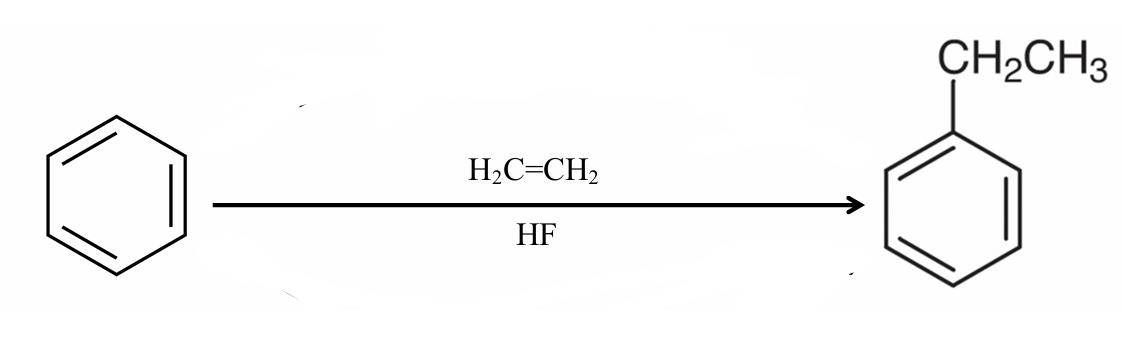
What reaction is this?
Direct Alkylation
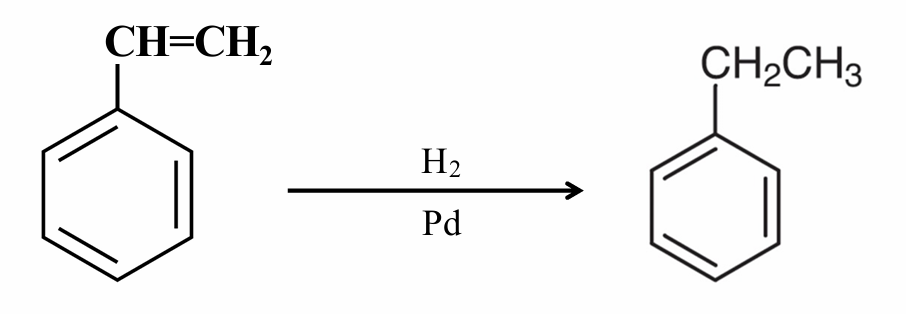
What reaction is this?
Side-chain Reduction
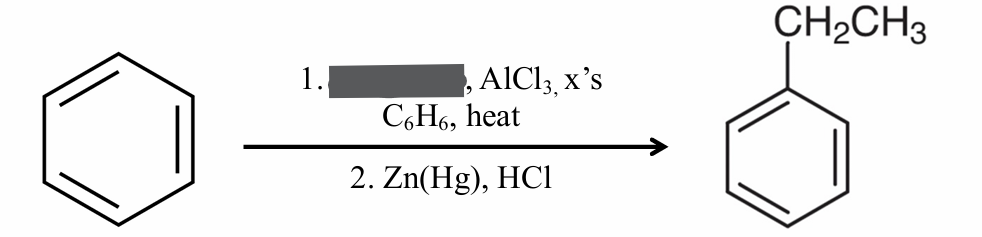
Identify the missing reagent
CH3COCl
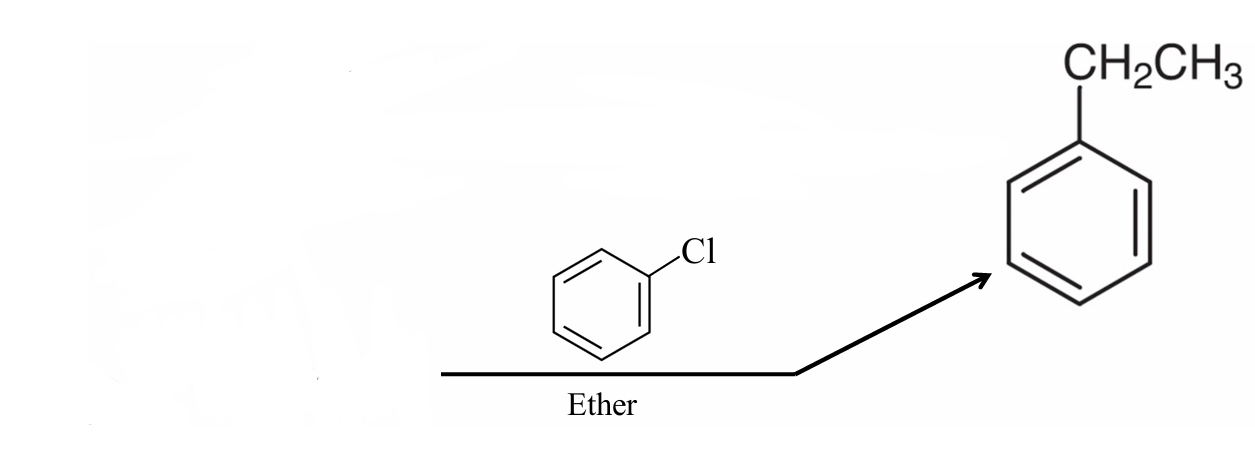
What should be the starting?
(CH3CH2)2CuLi
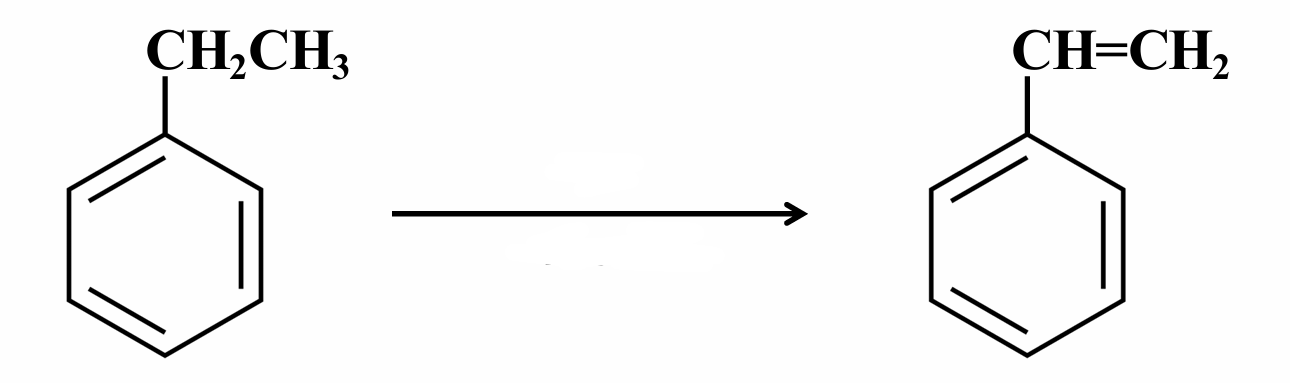
This is a dehydrogenation of an alkenylbenzene.
What are the missing reagents?
-H, Pt or Pd
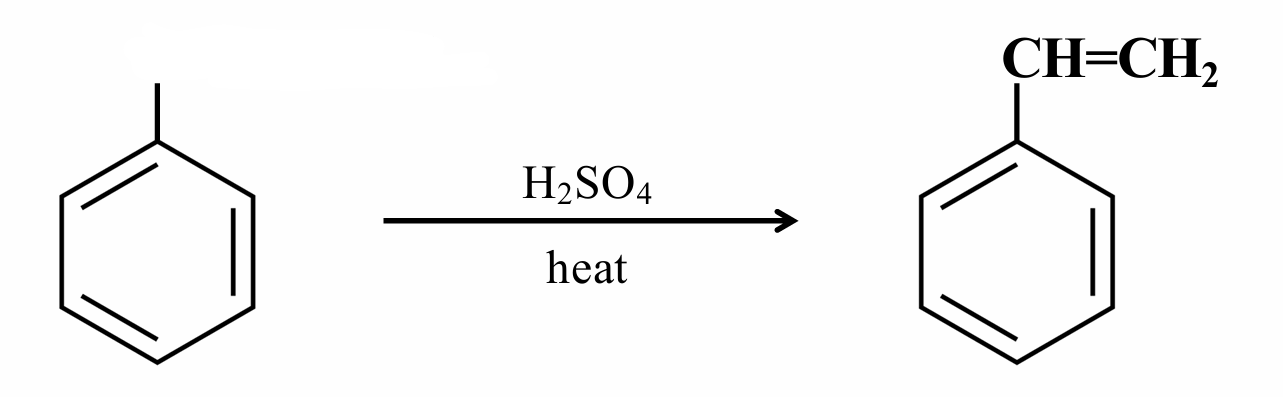
This is a dehydration reaction.
Identify the missing group on the reactant
1* Alcohol
CH2CH2OH
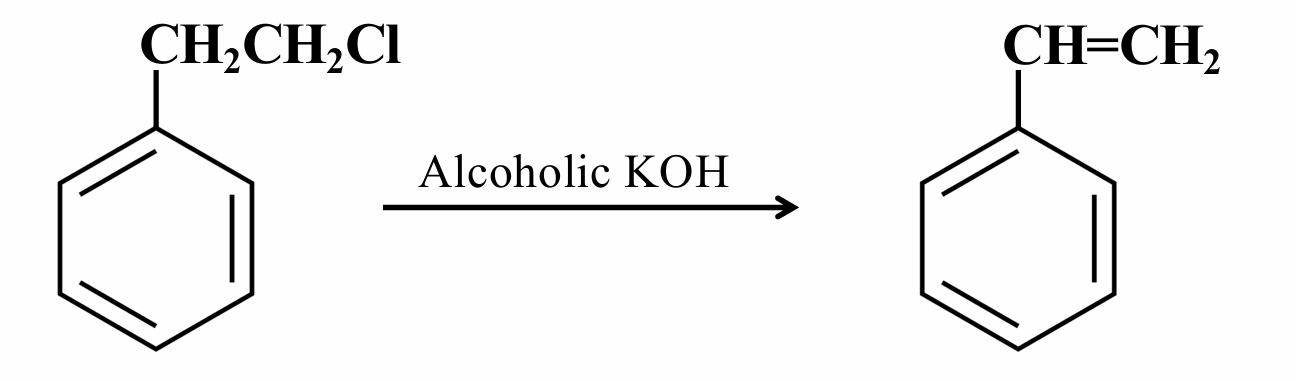
What reaction mechanism is this?
Dehydrohalogenation
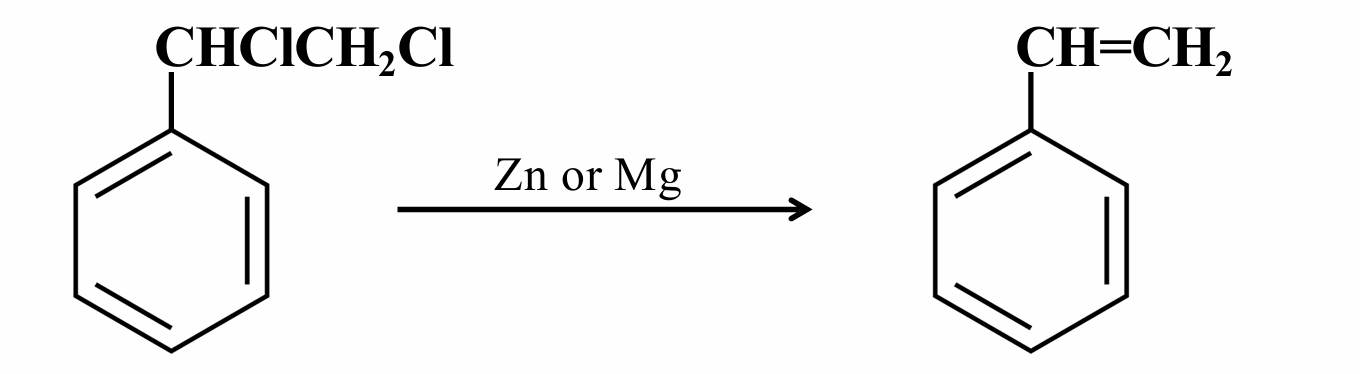
This is a Dehalogenation reaction. It is also known as
Simmon’ Smith Reaction
This is the aliphatic portion of arenes
Side chain
All alkyl groups present in the ring are converted to carboxyl groups, except tertiary alkyl groups. Why?
Because it has no benzylic hydrogen (the hydrogen attached to the carbon in the alkyl group).
Example: CH₃–C(–CH₃)₂
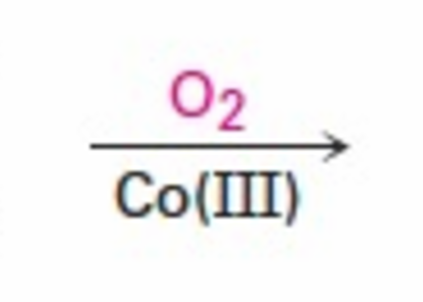
What reaction mechanism is this reagent used for?
Oxidation of dialkylbenzene
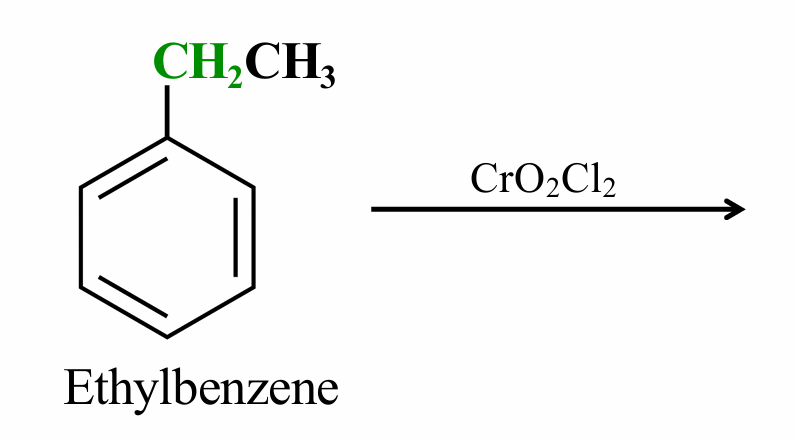
What would be the product of this oxidation reaction?
Acetophenone (C6H5)-COCH3
Side-chain bromination at the benzylic position occurs when an alkylbenzene is treated with
N-Bromosuccinimide (NBS)
The benzene ring carbon where an alkyl group is bonded can be converted to a carboxyl group by: followed by treatment with _
Ozonolysis; Hydrogen peroxide