NMR spectroscopy of keto-enol equilibrium
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
22 Terms
nuclear spin
protons have intrinsic magnetic moment from nuclear spin; typically I = ½, but dependent on atom
nuclear magnetic resonance (NMR) spectroscopy
interaction of the nuclear magnetic moment with external magnetic field; radio waves induce transitions between spin states
Energy of NMR interaction
E = -μ•B or E= -μz•B (when B is directed along Z axis)
(negative of magnetic moment (as a vector) multiplied by external magnetic field (as a vector))
E = hν
equivalent energies
magnetic moment of a proton
μ=γI
(magnetogyric ratio multiplied with spin angular momentum)
spin angular momentum
Iz= mIh*
discrete values
magnetogyric ratio
dependent on particle identity
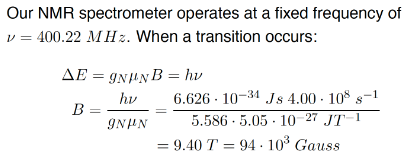
γh* = gNμN.
(nuclear g-factor multiplied by nuclear magneton)
nuclear magneton
e = elementary charge
mp = mass of proton
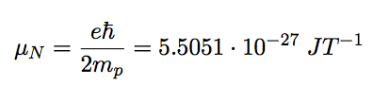
resonance frequency
dependent on chemical environment
Bloc=B(1-σ)
σ = shielding constant
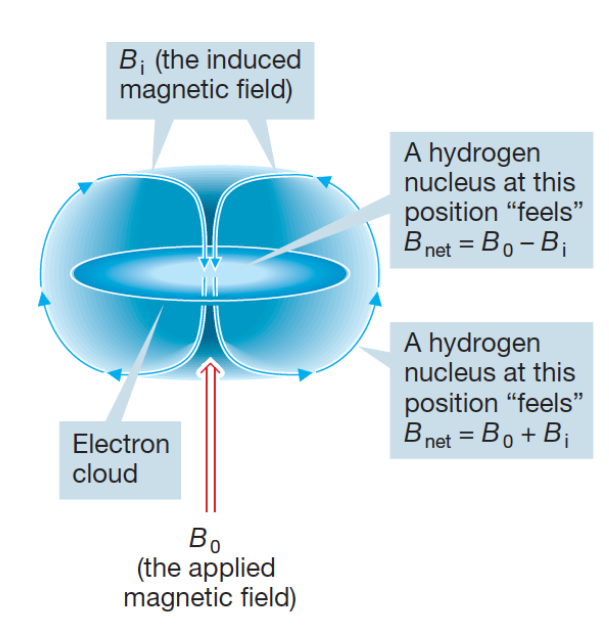
shielding constant (σ)
electrons in vicinity of proton “shield” it from the full effect of external magnetic field
NMR signal
provides:
Chemical shift (δ)
Integral
Coupling/multiplicity
Chemical shift (δ)
location of signal on x-axis; difference btwn resonance frequency and standard; measured in ppm; indepedent of external chemical field and frequency of spectrometer.
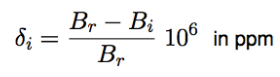
integral
determine relative number of hydrogens giving rise to the signal; area under the peak
Coupling/multiplicity
number of peaks in signal; spin of surrounding protons affects splitting of signals; distance between lines = coupling constant J (Hz)
tetramethylsilane/TMS/(CH3)4Si
standard for NMR against which chemical shifts are measured; most other protons are not shielded as well
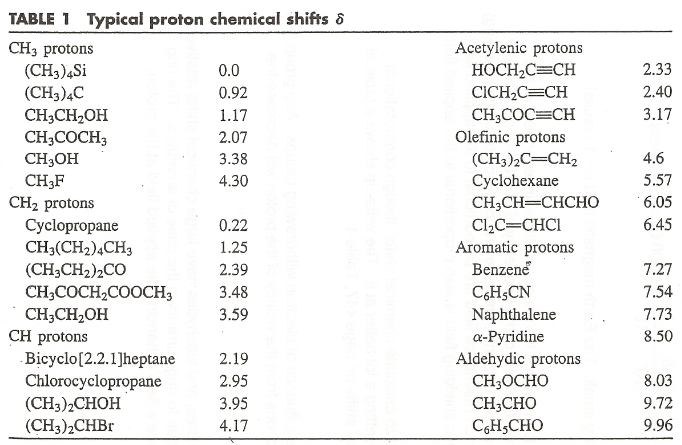
typical chemical shifts
aromatic ~ 7-8 ppm
olefin ~ 4-6 ppm
porphyrin can have negative sshift - enhanced shielding
Spin-spin splitting
neighboring protons affect resonance field; resonance frequency depends on spin orientation of neighbors; transmitted through bonding electrons
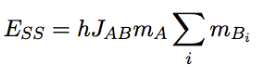
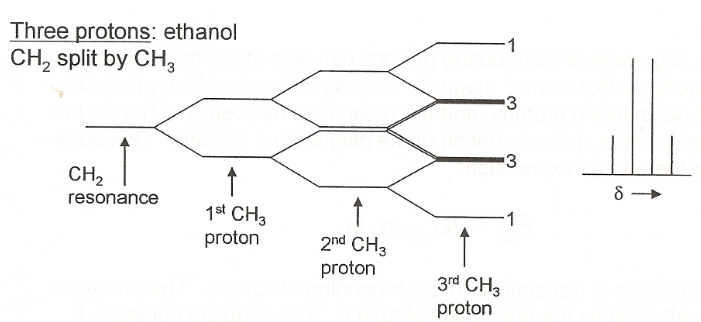
n+1 rules
how many lines from how many neighbouring protons that affect splitting
keto-enol equilibrium
spontaneous tautomerisation of ketones and alkene alcohols
Keq = [enol] / [keto]
![<p>spontaneous tautomerisation of ketones and alkene alcohols </p><p>Keq = [enol] / [keto]</p>](https://knowt-user-attachments.s3.amazonaws.com/30e832e1-2b2b-4d63-8bf4-f7b7b13f812a.png)
continuous wave NMR
radiofrequency held constant; mag. field scanned slowly; absorption as function of field strength
FT-NMR
stroong ext. mag. field that aligns protons along z-axis; strong radio pulse that rotaes magnetization into xy-plane (90°); produces oscillating RF signal, detected by receiver coil
Larmor frequency (ν)
how fast the proton precesses about the applied mag. field
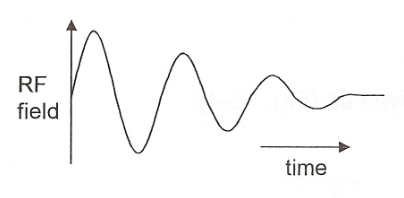
free induction decay
dampening of sinal over time; minimised via shimming (reduces heterogenity of applied mag. field) → slower dephasing + longer FID → better signal; Fourier transformed into peaks