Molecules and Cells: Exam 2 Content
1/101
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
102 Terms
Diffusion
movement of ions or molecules from an area of higher concentration to an area of lower concentration
Entropy:
the tendency of systems to reach a state of higher disorder or randomness
Osmosis:
movement of water across a cell membrane, from an area of higher concentration (low solute concentration) to an area of lower concentration (higher solute concentration)
When does osmosis occur?
1) two solutions are separated by a lipid bilayer
2) the solutions have different concentrations of dissolved ions and molecules
3) the dissolved substances can't move across the membranes
Concentration gradient:
a difference in a substance’s concentration in space—usually across a cell membrane
Potential energy:
the energy of position
Facilitated diffusion:
diffusion of substances across cell membranes through integral membrane proteins
Energy:
the ability to do work
selective permeability
two things that are important for substance to pass through lipid bilayer;
1) size
2) its charge
Passive transport
Movement of ions or molecules that does not require an input of energy. Passive transport occurs down a gradient and is spontaneous because it increases entropy.
Active transport
Movement of ions or molecules that requires an input of energy. Active transport occurs against a gradient and is nonspontaneous because it decreases entropy-for example, it makes the inside of a single-celled more organized than the non-life outside.
Channel:
An integral membrane protein that offers a "conduit" (or tunnel or pore) for a specific ion to cross the lipid bilayer passively, via diffusion.
3 points about channels
1) specificity - channels are specific, admit a single ion or molecule and nothing else
2) structure - channels have distinctive structure
3) few if any channels are open at all times, they have open and close configurations
Carrier:
An integral membrane protein that transports a specific molecule across the lipid bilayer passively, usually via diffusion, by changing shape once the molecule binds to it.
Key points about carriers
1) carriers are a mechanism for small molecules too large to cross liquid bilayers efficiently
2) each carrier transports a specific molecule
3) the movement of most of the transported substances occur via diffusion
key points about carriers
4) carrier activity like channel activity is regulated, they stop working if specific molecules called inhibitors bind to them
key differences between channels and carriers
carriers do not form an open channel for molecules to flow through the way channels do
Pump:
An integral membrane protein that transports specific ions or molecules across the lipid bilayer actively, often using energy carried by ATP, against their gradients.
Chromosome:
A DNA molecule — circular or linear, depending on the species — usually associated with specific proteins that bind to the DNA.
Ribosome:
A molecular machine consisting of RNA and proteins that is the site of protein synthesis
Organelle:
Any membrane-bound compartment inside a cell.
Cytoskeleton:
Rod-like proteins inside cells that function in structural support, transport of materials, and movement in some species.
Cell wall:
A stiff structure outside the cell membrane composed of carbohydrates and other molecules.
Flagellum:
A long, flexible structure that extends from the cell and whips or rotates to propel the cell through water.
Nucleus:
An organelle unique to eukaryotes enclosed by a double membrane and containing the cell's chromosomes.
Mitochondrion:
An organelle found in all eukaryotes that functions in "burning" sugars to provide energy for the cell.
Eukaryote:
A lineage of species with cells that contain a nucleus and mitochondria.
Prokaryote:
A species in the lineages Archaea and Bacteria, with cells that lack a nucleus.
Vacuole:
An organelle that stores key molecules; very prominent in most plant cells.
Chloroplast:
An organelle that contains the molecular machinery required for photosynthesis.
Cell wall:
A stiff structure outside of many types of cells, usually made primarily of carbohydrates.
Endoplasmic reticulum (ER):
A eukaryotic organelle that forms a branching network of narrow tubes and flattened sacs. The place where proteins, membrane lipids, and other molecules are synthesized and/or processed. Can have ribosomes associated with it (rough ER) or not (smooth ER).
Golgi:
An organelle comprised of stacked, flattened sacs, where proteins and other molecules are processed and packaged for shipment to specific destinations.
Actin filaments:
Small-diameter cytoskeletal fibers made of subunits of the protein actin. responsible for movement
Intermediate filaments:
Cytoskeletal fibers whose size is intermediate between those of actin filaments and microtubules and can be made from a variety of protein subunits.
Microtubules:
Large-diameter, hollow cytoskeletal elements made of pairs of the proteins α-tubulin and β-tubulin.
Endomembrane system:
A collection of machines, cytoskeletal components, and organelles that together produce, process, and transport proteins and lipids destined for organelles, the cell membrane, or outside the cell.
Signal sequence:
A series of amino acids at the start of a protein that allows that protein to enter the endomembrane system.
Motor protein:
A protein that functions in cell movement through shape changes caused by phosphorylation or dephosphorylation.
Phosphorylation:
The addition of a phosphate group (PO43-) to a protein or other molecule. In most cases, the phosphate group comes from ATP. Dephosphorylation is the reverse process.
putting the endomembrane system together 1-5
1) mRNA is exported from the nucleus, through a nuclear pore
2) protein synthesis ( linking amino acids via peptide bonds) begins in a ribosome
3) a single sequence on the new protein binds to an RNA-protein particle which interacts with a receptor on the ER membrane
4) the protein enters the inside of the ER, through a channel
5) folding and other processing occurs inside the ER
putting the endomembrane system together 6-9
6) processed
Which statements accurately describe the differences between bacteria and archaea?
The cell membranes and cell walls have different lipid and carbohydrate compositions and can be used to differentiate the archaea from bacteria.
which organelles have double membrane
mitochondria, chloroplasts, nucleus
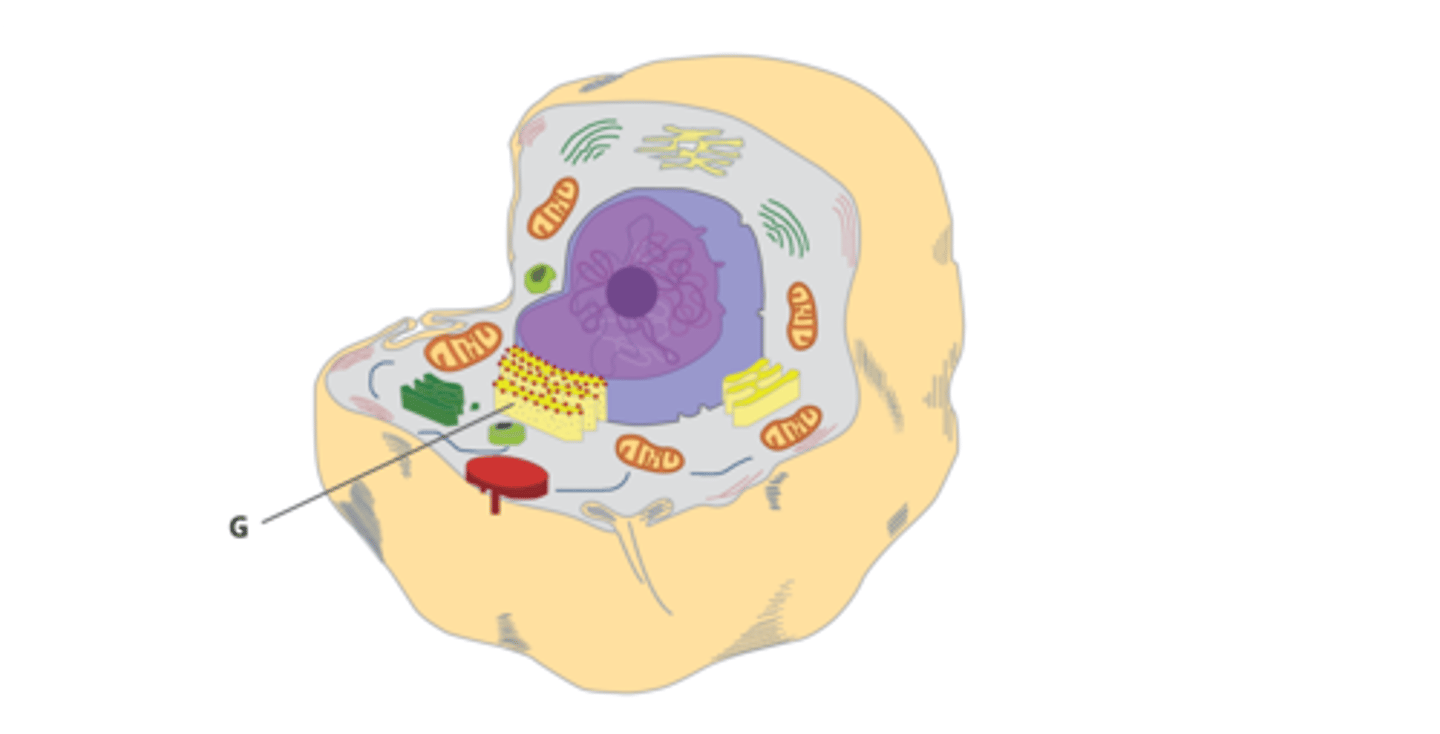
The organelle identified by the letter G in this image serves what function in the cell?
Membrane and secretory proteins are made on the ribosomes of the rough endoplasmic reticulum.
Which statement is not accurate comparisons of plant and animal cells?
animals cells also have cell wall ( NOT TRUE)
Which cytoskeletal elements have the most diversity in terms of protein composition?
Intermediate Filaments: This class of cytoskeletal elements, classified only by its intermediate size, is the most diverse in terms of protein type.
Each flagellum is composed of the arrangement of tubulin subunits, organized into a "9 +2" pattern. Which type of cytoskeletal element would be associated with these flagella?
Flagella contain microtubules organized in a 9 + 2 pattern
Many cells move across surfaces by "crawling"; in fact, this is the main way that cells move in multicellular animals. Cell movement is accompanied by changes in the shape of the cell, and these changes are mediated by reorganization of the cytoskeleton. Which cytoskeletal component is typically associated with cell movement?
Actin filaments are the main cytoskeletal element involved with movement.
Which structure is required for proteins to enter the endomembrane system?
The signal sequence at the start of a protein is what allows it to enter the endomembrane system for processing and packaging.
how proteins enter the endoplasmic reticulum. Place the first event at the top and the last event at the bottom.
1) mRNA is exported from nucleus and associates with ribosome's in the cytoplasm
2) signal sequence of new protein binds to RNA protein complex
3) RNA-protein complex guides ribosome complex to the ER membrane
4) protein is inserted into the interior ( lumen) of the ER
5) signal sequence is removed
Imagine you are investigating cells that are not processing proteins correctly. Upon closer examination, you find that the proteins are missing specific sugar groups. Based on this information, what part of the endomembrane system is defective in these cells?
Most glycosylation of proteins occurs in the cis and medial regions of the Golgi.
Why is it appropriate to call an active site "active?"
it's the location where chemical activity—a reaction—takes place
Enzymes make nonspontaneous reactions go
False.
Enzymes only influence the rate of spontaneous reactions; nonspontaneous reactions can't occur at all, enzymes or no enzymes.
important info about enzymes 1-3
1) They are each specific to one reaction;
2) They each contain an active site where reactants bind and where the bond-breaking and bond-making required for the reaction actually occur;
3) Enzymes speed up reactions by bringing reactants together in an orientation that allows them to interact and then lowers the free energy present in the transition state;
important info about enzymes 4-5
4) Although the enzyme is intimately involved in the reaction, it is the same molecule before the reaction starts and after it ends
5) Enzymes do not affect the amount of free energy in reactants or products.
Endergonic reaction:
One that results in an increase in free energy; another way of referring to a nonspontaneous reaction.
Exergonic reaction:
One that results in a decrease in free energy; another way of referring to a spontaneous reaction.
Activation energy:
The amount of energy required to get a chemical reaction through its transition state.
Transition state:
During a chemical reaction, an intermediate state where old bonds are being broken but new bonds have not yet formed.
Enzyme:
A protein that catalyzes a chemical reaction.
Active site:
The place on an enzyme (or ribozyme) where a reaction is catalyzed.
what can
1) inures probability that reactants encounter each other in correct spacial orientation
2) Lower activation rates
enzymes
why aren't spontaneous reactions fast
1) spacial orientation
2) activation energy
If you created reaction mixes in test tubes that contained everything required to make the peptide bonds to form proteins, the phosphodiester bonds to make RNA and DNA, and the glycosidic bonds to make carbohydrates--but no source of energy--would the reactions take place spontaneously or would they be nonspontaneous?
Nonspontaneous. All of these reactions, along with the reactions that form phospholipids and most other cell components, require an input of energy.
Cells are highly organized entities, filled with large molecules and complex molecular machines. In terms of free energy, then, it is logical to claim, "Life is nonspontaneous."
True. Every cell and every organism needs energy to stay alive and reproduce.
Due to the close proximity of so many full negative charges, ATP has extremely high potential energy. Why do the presence of these charges increase potential energy?
The like charges repel each other, pushing the electrons involved out and away from the positive charges in nuclei. This give the molecule extremely high potential energy.
If a protein gets phosphorylated, what happens to it relative to the ability to do work?
The additional negative charges repel full and partial negative charges in R-groups, raising the protein's overall potential energy.
Electrical energy:
Energy related to interactions among charged particles.
Thermal energy:
The energy of motion in ions and molecules, measured as temperature.
Potential energy:
Energy that is related to an object's position.
Chemical reaction:
Conversion of substances (ions or molecules) into other substances via breaking and forming chemical bonds.
Free energy:
The total energy available to do work—a combination of entropy and thermal and potential energy.
Energetic coupling:
A phosphorylation reaction that makes a nonspontaneous reaction spontaneous, because it raises the free energy of the reactants.
Compared to the electrons in nonpolar C-C and C-H bonds, do the electrons in C-O bonds have higher or lower potential energy?
Lower. This is key. When electrons are closer to a nucleus, they have lower potential energy and are more stable.
Recent work has shown that as electrons flow through complexes I, III, and IV, the machines change shape in ways that allow protons to move from one side of the inner mitochondrial membrane to the other, establishing a much higher concentration of H+ inside the mitochondrion's tubules and sacs than outside. This change from chemical energy (in redox reactions) to mechanical energy (as the complexes move like pumps), is an example of an energy transformation.
True.
Before the ETC, the energy carried in the electrons that NADH and FADH2 donate to complexes I and II existed in chemical bonds, so were an example of chemical energy. The mechanical energy of proton pumping is a different form of energy.
As an electron acceptor, oxygen also completes the overall redox reaction that occurs in cellular respiration. At the end of the process, the carbon atoms in glucose are oxidized, forming CO2, and the oxygen atoms in O2 are reduced, forming water.
True.
Every oxidation event, or loss of an electron, has a corresponding reduction event, or gain of an electron.
The addition of a phosphate group to ADP to form the product ATP is a spontaneous reaction on its own.
False. Adding more negative charges to ADP increases electrical repulsion and increases potential energy, and thus free energy. The increase in free energy comes from the kinetic energy of protons passing through ATP synthase.
ATP synthase is considered an enzyme because it 1) is a protein, 2) catalyzes a reaction--specifically the addition of a phosphate group to ADP to form ATP, via the mechanical twisting force generated in the F1subunit, and 3) is unchanged after the reaction.
True.
The mechanical force generated by the machine's rotor is an addition of free energy that drives an otherwise non-spontaneous reaction.
ATP synthase uses mechanical force to generate ATP, which involves formation of a new chemical bond. This is an example of an energy transformation.
True.
Here's an analogy to the energy transformation that occurs in ATP synthase: the mechanical energy of water flowing through a dam makes turbines spin, which then generates electricity.
Redox (reduction-oxidation) reactions:
Chemical reactions involving the gain (reduction) or loss (oxidation) of an electron.
Cellular respiration:
Any method for producing ATP that includes an electron transport chain.
Electron transport chain (ETC):
A series of machines that uses an electric current to pump protons across a membrane, establishing a proton gradient that is then used to generate ATP. When the ETC uses oxygen as an electron acceptor, ATP production via the combined action of the ETC and ATP synthase is called oxidative phosphorylation.
What does the ETC create?
a high concentration of protons with positive charges inside the mitochondrion. this high conc. of protons creates a strong conc. gradient that favors movement of protons from inside the membrane back outside
ATP synthase:
The multi-protein machine that transforms the kinetic energy in a flow of protons to mechanical energy that catalyzes the addition of a phosphate group to ADP to form ATP.
Glycolysis:
A sequence of ten enzyme-catalyzed reactions that begins with glucose and ends with pyruvate, producing 2 ATP and 2 NADH per molecule of glucose.
Pyruvate processing:
A series of enzyme-catalyzed reactions that begins with pyruvate as a substrate and produces acetyl-CoA and NADH.
Citric acid cycle:
A sequence of nine enzyme-catalyzed reactions that begins with acetyl-CoA, completes the oxidation of glucose to CO2, and produces ATP, NADH, and FADH2.
NADH, FADH2, Q:
Molecules that function as electron carriers during cellular respiration, meaning that they transport electrons to or within the electron transport chain.
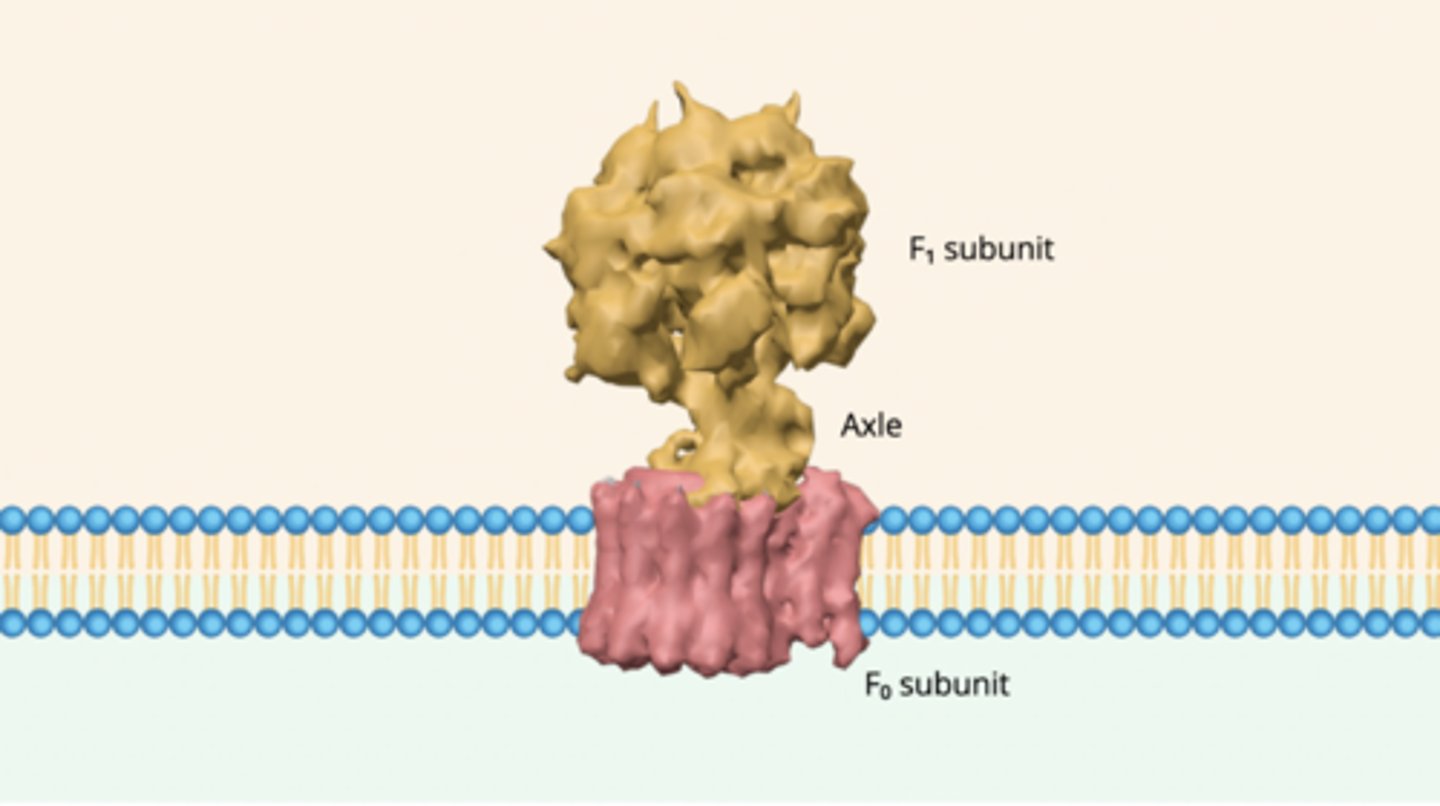
Molecular machine: ATP Synthase
1) protons enter the flow through the F(0) subunit, making the base spin like a router
2) the axle attached to the rotor spins in response
3) the twisting force generated by axle makes F(1) subunit change shape in a way that catalyzes the addition of a phosphate group to ADP, forming ATP
oxidation phosphorylation
1) The ETC which produces a proton gradient
2) ATP synthase, which uses proton gradient to produce ATP. The term is appropriate because oxygen is used as final electron acceptor in the ETC that generates the proton gradient
In the reactions that occur during the glycolysis, pyruvate processing, and citric acid cycle stages of cellular respiration, NAD+ and FADH are oxidized.
False.
NADH and FADH2 are the reduced forms of NAD+ and FADH, respectively.
Which statements accurately describe the process of glycolysis?
- Glycolysis has a net yield of 2 ATP, because 4 ATP are produced but 2 ATP are invested.
- Glycolysis starts with an "investment phase" that adds phosphate groups to 6-carbon sugars and uses 2 ATP.
- In reaction 6, an isolated phosphate group, not one that is part of ATP, is added to a 3-carbon molecule.
When pyruvate acts as an electron acceptor during fermentation in your muscles, it is reduced and NADH is oxidized, yielding lactic acid and NAD+.
True.
Since pyruvate gains electrons and NADH loses electrons, this is a redox reaction.
When oxygen is depleted in your muscles and cellular respiration shuts down, fermentation begins so ATP production can continue. How much ATP does fermentation produce per glucose molecule compared to the amount of ATP produced by cellular respiration per glucose molecule?
~5%.
Fermentation is better than nothing in terms of keeping muscle cells alive, but it is extremely inefficient compared with cellular respiration.
If your muscles become depleted of oxygen after strenuous exercise and switch to fermentation, you can continue activity for a short while. But then you will need to rest (or you will collapse). As you rest, oxygen levels will build back up. When this happens, the lactic acid that has built up during fermentation is converted back to pyruvate. What happens to this pyruvate, now that O2 is again available?
Cellular respiration resumes because the pyruvate is processed to feed the citric acid cycle and ETC.
When this happens, ATP levels will rise and you will start to feel better.
In general, species that do not perform cellular respiration at all and rely exclusively on fermentation are much more physically active than species that perform cellular respiration.
False.
Fermentation only produces about 1 ATP for every 20 ATP produced in cellular respiration, so organisms that only perform fermentation, or obligate fermenters, have much less energy available than species that do aerobic respiration.
The phrase "Cells respire or expire" may be true for many organisms, but not for those that only use fermentation to stay alive.
True.
Obligate fermenters do not respire, but they do not expire (die immediately).Fermentation: A pathway that transfers electrons from NADH to a carbon-based molecule to regenerate NAD+and keep glycolysis running to produce small amounts of ATP.
Fermentation:
A pathway that transfers electrons from NADH to a carbon-based molecule to regenerate NAD+and keep glycolysis running to produce small amounts of ATP.
Aerobic respiration:
Cellular respiration that uses O2 to accept electrons from the electron transport chain and produces water as a byproduct.