FOSC201 - Water & Carbohydrates
1/83
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
84 Terms
What is water?
2 hydrogens + 1 oxygen, polar covalent bonds, 3 states (solid, liquid, gas), 70% of earths surface.
Importance of water (% in foods)
Meat = 50-80%
Fruit = 75-85%
Vegetables = 75-95%
Determines
physiochemical characteristics (texture, shape, stability, storage ability.)
quality
reactions
degradation/shelf life
Water is polar (electrons unevenly distributed) which allows…
Hydrogen bonding - responsible for special properties
Cohesion
Water binding to itself
Unusual properties of water
High
melting pt
boiling pt
heat capacity
surface tension
Because of high amount of energy to break hydrogen bonds
Solid water
Becomes more dense until 4 degrees then becomes less dense (ice floats on water).
Molecules move slower - easier to form hydrogen bonds and form a crystalline structure - water molecules held further apart (volume increases).
Liquid water
Molecules mobile - hydrogen bonds form so relatively close together/semi-ordered (each water bonds to 4 others)
Molecules slide past each other.
Gas water
Hydrogen bonds broken (a lot of energy required)
Molecules move fast and far apart (no time for hydrogen bonds to form)
Heat of vaporization
Energy required to change liquid into gas
Vapour pressure/relative humidity
Pressure exerted by a vapour above its solid or liquid state.
Increases with temperature.
Inversely related to intermolecular forces (strong H bonds in water = lower vapour pressure)
Water boils when…
vapour pressure > atmospheric pressure
increase pressure = higher boiling pt
decrease pressure = lower boiling pt
Water phase diagram
Describes behaviour/states of pure water according to temp and pressure
Triple pt (below 1atm): all three phases at equilibrium
below triple pt no liquid
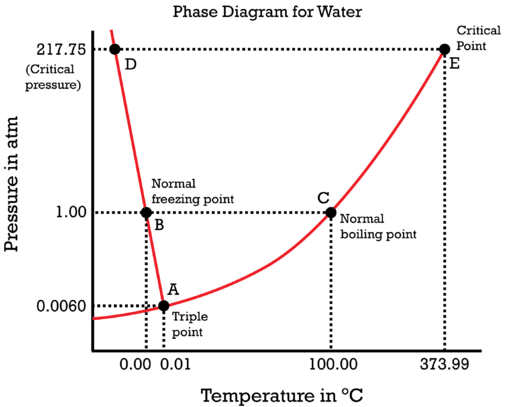
Freeze drying (requires…)
Low temp - below triple pt
Low pressure
Ice sublimes to gas
Slow & expensive
Water droplets form due to…
Surface tension
resists external force
fluid acquires least surface area possible
Stronger bonds at surface
Water rises in hollow tube due to…
Capillary action/adhesion
forces between different molecules
water adhesion to vessel wall - climbs
concave meniscus
narrower tube = greater effect
Solute effects on water properties
Colligative properties
Decrease melting pt
increase boiling pt - more energy needed to overcome atmospheric pressure
decrease vapour pressure (more energy needed to be greater than atmospheric pressure)
Decrease freezing pt
Change Aw
Moisture content is…
Total quantity of water in a food
% as wet weight = 100 x weight of water/fresh weight of food
% as dry weight = 100 x weight water/weight dry solids
Water content determined by…
Drying to constant weight
oven
microwave
vacuum
Water activity (Aw) - definition
Ratio of vapour pressure of water in a food to saturated vapour pressure of pure water
Free energy of water/ fraction of water available
Aw determines
Microbial growth
Rate of chemical reactions
Food stability/ shelf life/ functional properties
Relationship between Aw and moisture content described by…
Moisture sorption isotherm
predict stability of a food
depends on food comp and enviro conditions (temp, humidity)
goes from high stability to fresh/perishable foods
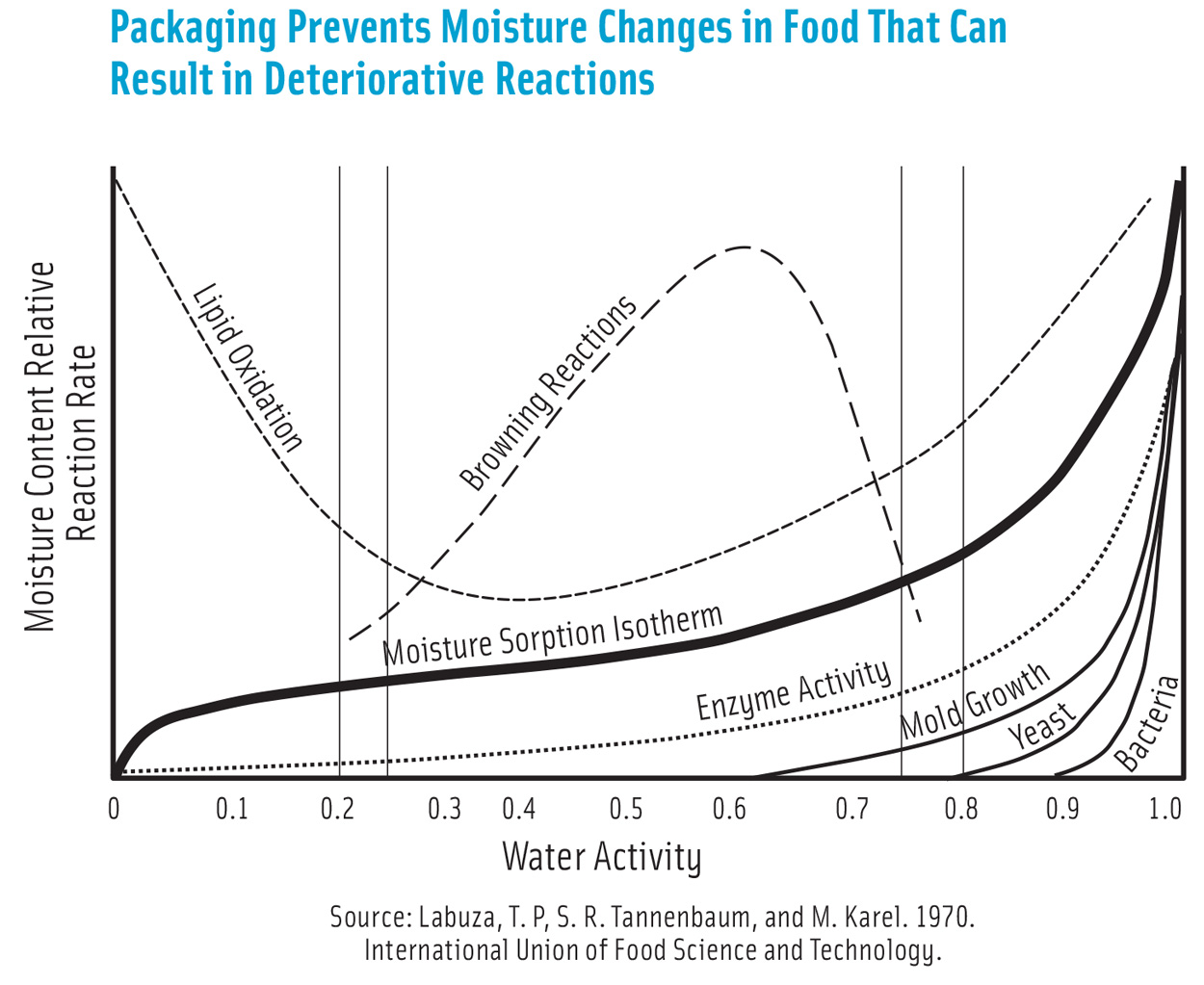
Zone 1 (moisture sorption isotherm)
High stability
Tightly bound water (not available) - chemically bound
Immobile
Aw = 0 - 0.25
Zone 2 (moisture sorption isotherm)
Capillary water (filling in gaps in product)
Loosely bound
Reduced availability
Some mobility
difficult to freeze
Aw = 0.25-0.75
Zone 3 (moisture sorption isotherm)
Free water
Fully available/ mobile
available for enzymatic and chemical reactions and microbial growth
Freezing
Lowing binding energy - easy loss
Aw = 0.75-1
Aw<0.6
No microbial activity
Aw<0.7
most fungi and moulds inhibited
Aw<0.8
Most yeasts inhibited
Aw<0.9
Most bacteria inhibited
Enzyme activity and Aw
Zone 1: no enzyme activity due to low substrate mobility
Zone 2: slow rate of reaction (limited mobility)
Zone 3: rate increases rapidly
Lipid oxidation and Aw
Zone 1: high - free radicals (autoxidation)
Zone 2: decreased (chelating agents and antioxidants)
Zone 3: high - antioxidants diluted (Hydrolytic rancidity)
Browning and Aw
Zone 1: negligible (low mobility, sugar and amino acids are water soluble)
Zone 2: increase (mobility and high conc of reactants)
Zone 3: decrease (high moisture suppresses reaction and dilutes reactants)
Functionality and Aw (impact of characteristics)
Aw = effective water conc for hydration of materials
Impacts on characteristics
texture
soft vs hard
moist vs dry
crunch/crisp
Moisture migration (determines)
From high Aw to low Aw
Determines
moisture absorption (hygroscopicity - low Aw foods will undergo)
Moisture loss (dehydration/drying - high Aw foods will undergo)
Driving force for moisture migration is…
Water activity
Causes
microbial growth, degradation
texture/sensory change
To avoid
separate package
edible barriers
modify Aw with salt, sugar, humectants
Aw measured using…
Aqualab water activity meter
Dew point hygrometer cooled until dew point
Find relative humidity → calculate Aw
Temperature and Aw
Aw increases with temp (at constant moisture)
stability at lower temps
Non reversible structural changes cause…
Hysteresis
collapse during drying
incomplete dehydration/absorption
Adsorption isotherms: hygroscopic products
Desorption isotherms: drying products
Solutes and Aw
Decrease Aw (solutes interact with water)
Properties determined by number of solutes in water
Colligative properties
decrease melting pt
decrease vapour pressure
increase boiling pt
alter Aw
Raoult’s Law
In ideal conditions gives Aw, states when solute added vapour pressure decreases and affects Aw.
correction factor y(gamma) used because solutes interact with each other as well
larger molecules have greater affect on Aw (salt)
Carbs are…
most abundant organic compound in nature
made of C,H,O
polyhydroxy aldehydes (C=O on end)
polyhydroxy ketones (C=O in middle)
Determine functional properties of food
Biological functions of carbs
Energy source
Structural components (cellulose)
Biomolecules (DNA)
Nutritional functions of carbs
50-55% energy intake
overconsumption → obesity
Functional properties of carbs in food
Sweeteners
Precursors for flavour and odour (caramelisation and maillard reaction)
Carb classification (2 things)
Type of saccharide (Triose, pentose, hexose)
Number of saccharides (mono, di, tri, oligo, poly saccharides)
Optical activity of carbs (isomers)
optical isomers - chiral carbon
Rotation of polarised light
left L isomer
Right D isomer (predominant form in food)
Structure of carbs vary on
number of carbons in a unit
Position of C=O, OH
ring structures (furanose - 5 atom, pyranose 6 atom)
number and type of saccharides
Position of hydroxyl group in relation to C=O in carbs determines
Cyclic isomer
a (OH below O from C=O)
B (OH above O from C=O)
Reducing sugars are…
Sugar with an available carbonyl group capable of acting as a reducing agent (important for reactivity)
glucose
galactose
maltose
fructose
Caloric value of carbs
Important energy source
3.75Kcal/g
Digestibility of carbs
Available - digested and absorbed in small intestine, hydrolysed to monosaccharides by enzymes.
Partially available - lactose, depends on existence of digestive enzymes
Unavailable - dietary fibre
insoluble fibre = cellulose, lignin
soluble fibre = gums, fructans, pectins, pentosans
resistant starch
Sensory properties of carbs
Mono, di and oligo are sweet
Oligo sweetness decreases with increasing carbon number
Precursors for flavour and colour
caramelisation
maillard reaction
Monosaccharides occur as…
Pentoses (5 carbon)
D-Xylose (wood sugar - corn, bran, cherry, peach)
L-Arabinose (pectin sugar - beets, gum arabic)
D-Ribose (nucleic acids, vitB2)
Hexoses (6 carbons)
D-glucose (aldose sugar - most abundant monosaccharide)
D-Fructose (ketose sugar - in fruits and honey
D-Galactose (lactose in milk)
D-Mannose
Glucose, galactose and mannose different due to…
position of OH (same chemical structure)
Disaccharides are…
2 monosaccharides joined by glycosidic bonds (condensation reaction - bond between carbonyl group and hydroxyl group)
Disaccharides - maltose
Glucose + glucose
a-1,4 glycosidic bonds
reducing sugar
Disaccharides - sucrose
Glucose + fructose
a-1,2 glycosidic bonds
non reducing (reducing ends of bond monosaccharides bonded)
Disaccharides - lactose
galactose + glucose
B -1,4 glycosidic bonds
reducing
Trisaccharides - maltotriose
3x glucose
a-1,4 glycosidic bonds
reducing
Trisaccharides - Raffinose
galactose + glucose + fructose
non reducing
a-1,2 and a-1,6 bonds
Polymers of monosaccharides…
polysaccharides (joined by glycosidic bonds)
linear
branched
amorphous
tasteless
colourless
viscous
Homopolysaccharides
cellulose
starch
glycogen
inulin
Homopolysaccharides - cellulose
Glucose (chains can H bond with each other to form fringed micelles)
B-1,4 glycosidic bonds
Cell wall - structure
indigestible
Homopolysaccharides - starch
Glucose - linked by a glycosidic bonds
different proportions of
amylose - linear (a-1,4), helical structure
amylopectin - branched (a-1,4, a-1,6 at branches every 25 glucose units)
Homopolysaccharides - glycogen
Branched chain polymer of glucose like amylopectin
a-1,4, a-1,6 at branches
branches every 12-18 glucose units
animal storage reserve for glucose and energy
Homopolysaccharides - inulin
Fructose polymer
B-1,2 bonds
unavailable carb - not digested
Heteropolysaccharides - mucilages
agar, alginates, carrageenan
gel forming
Heteropolysaccharides - Gums
Hexose + pentose + uronic acid
gum arabic
thickener, stabiliser, emulsifier
Heteropolysaccharides - pectins
a D-galacturonic acid (oxidation of D-galactose)
middle lamella of cell walls
Polysaccharide functionality (water interactions)
High affinity for water - form H bonds
Swell and hydrate
normally soluble (not cellulose)
Amylose and amylopectin arranged radially in alternating layers
Starch granule
Gelatinisation (occurs when…/results in…)
starch granules heated with water so swell as water is absorbed
Amylose leaches and creates viscous paste
Too much heat leads to implosion and complete dissolution
Gel may form when cooled
Gelation is…
process of forming a gel
water trapped between polymers
Occurs upon cooling after gelatinisation
More amylose = stronger gel
Double helices and egg box model are…?
Models of gelation
Reactions of carbs
chemical
oxidation (loss of electrons) reduction (gain of electrons)
condensation
dehydration
isomerisation
non enzymatic browning
caramelisation
maillard
Reactions of carbs - oxidation
Aldehyde to aldonic acid
Uronic acids
used for blood glucose measurement
Reactions of carbs - reduction
Monosaccharide → sugar alcohols
sugar substitutes for diabetics
Reactions of carbs - condensation (loss of water)
2 molecules combine - polymerisation
Reactions of carbs - dehydration (condensation reaction)
intramolecular condensation - loss of water and bond formation within one molecule
Furfural
HMF
Reactions of carbs - isomerisation (starts with e)
Conversion of monosaccharides into other monosaccharides with same chemical formula
Enolisation
1,2 - enolisation (acid)
2,3 and 3,4 enolisation (alkali)
Non-enzymatic browning generate…
Brown colours and flavour compounds in food (caramel, toffee, nutty)
caramelisation
maillard reaction
Caramelisation is…
Pyrolysis/thermal degradation of sugar at high temp
Generates a-dicarbonyl which can react to give furfural and HMP (undesirable)
low pH = colour
neutral pH = colour and aroma
Maillard reaction
reducing sugar and amino acid → glycosylamine
polymerisation of molecules →colour change
generates a-dicarbonyl like caramelisation
Strecker degradation - maillard reaction
a-dicarbonyls react further with amino acids → strecker aldehydes + amino ketones
strecker aldehydes are aroma active
Amino ketones → pyrazines (roasted, nutty flavour)