ANSC*3080: Endocrine System
1/106
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
107 Terms
endocrine
Refers to a hormone that is:
Produced and secreted within the body.
Opposite of exocrine, which is secreted outside body (mammary bland, lumen of digestive tract, etc…).
Acts at a distance from the release site.
paracrine
Acts on nearby cells.
autocrine
Acts on its own secreting cell.
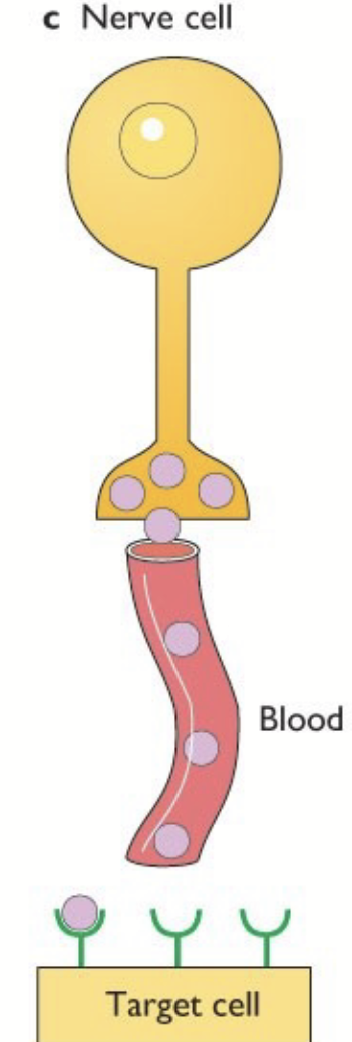
neuroendocrine
Synthesized by nervous tissue and carried in the blood. Neurohormone released by neuron and transferred in blood.
gland
Cluster of cells organized for synthesis/release of a compound. Can be endocrine or exocrine.
hormones
Regulatory chemicals produced in an endocrine gland or scattered cells (e.g. adipose tissue), secreted into the blood and carried to its target cell that responds by altering its metabolism.
Involved in maintaining homeostasis.
Subjected to tight regulation by feedback from target organs.
Regulation of synthesis and mode of action differ greatly based on their structure.
feedback control
Cyclic systems (loops) that control the amount of hormone released.
Circulating hormones from the endocrine glands provide negative feedback both to the hypothalamus and pituitary. Serves to regulate the secretion of hormones.
Two major loops: short loop and long loop (most prominent).
steroid hormones
Derived from stepwise conversion of cholesterol by multiple enzymes.
Lipid-soluble = leave production cells and enter target organ by diffusion through the membrane.
peptides, proteins, glycoproteins
Chains of amino acids → sequence determines the primary structure and nature. Genetic code = 3 base sequence code for one AA.
These hormones must be transcribed and translated.
DNA → transcription (nucleus) → mRNA → translation (ribosome/cytosol) → protein
After translation, protein can be modified: glycosylation (CHO chains attached), phosphorylation.
Modified, packaged (because water-soluble) in the ER and the Golgi. Secreted by exocytosis.
amino-acid derivatives
Generally derived from tyrosine and tryptophane (e.g. thyroid hormones, catecholamines - dopamine, adrenaline).
fatty acid derivatives/eicosanoids
Derived from cell membrane phospholipids via enzymatic modification (arachidonic acid). Produced locally, have mainly autocrine and paracrine effects (transported very quickly).
e.g. protaglandins, prostacyclins, thromboxanes
pineal gland
On the roof of the third ventricule, encapsulated by meninges. Secretes melatonin.
Under the indirect influence of the hypothalamic circadian center.
Major role during sleep patterns and recognition of seasons.
Melatonin secretion is stimulated during dark phases.
melatonin
Amino acid derivative secreted by the pineal gland, under the indirect influence of the hypothalamic circadian center.
Major role during sleep patterns and recognition of seasons. Stimulated during dark phases.
In mammals: signal comes from the eye. Can supplement to regulate sleep/wake cycle - tricks body into thinking it’s dark.
gastrin
Secreted by the stomach wall, leads to stimulation of acid secretion.
secretin
Secreted from the small intestine, stimulates the pancreas.
cholecystokinin
Secreted by small intestine, stimulates pancreas and gallbladder.
gastric inhibitory peptide
Secreted by small intestine, inhibits stomach activity.
renin
Secreted by kidneys. Increases aldosterone secretion by the adrenal cortex.
erythropoietin
Secreted by kidneys. Increases production of RBCs in bone marrow.
stomach wall
Releases gastrin.
small intestine
Releases secretin, cholecystokinin, gastric inhibitory peptide.
kidneys
Release renin and erythropoietin.
adipose tissue
Release leptins and other adipokins.
leptins
Receptors can be found in the hypothalamus. Give information about energy storage status; regulate appetite.
hormone actions
Hormones are secreted in the blood and act at a distance from the release site. They need to travel in the blood (solubility), survive long enough, and be active at the target site.
Hormones trigger specific actions in specific target cells. Requirement for specific recognition: RECEPTORS.
Principle of action is based on hormone biochemical structure and properties.
receptors
The key to hormone action! Hormone binding initiates the effects
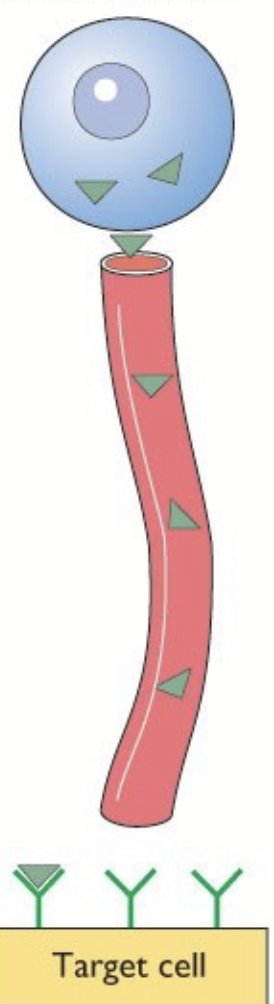
lipophilic hormones
Diffuse out of producing cells. Circulate mainly bound to carriers in blood. Diffuse in target cells → intracellular receptors.
e.g. steroid and thyroid hormones.
Insoluble in water, circulate associated to carrier proteins. Some carriers are specific = globulins (CBG, DBG, etc.). Some are non-specific (albumin and prealbumin).
A small portion remains free and diffuses to the tissues. Free hormone is the active portion, but is also susceptible to degradation.
Free form (active) is involved in feedback loops.
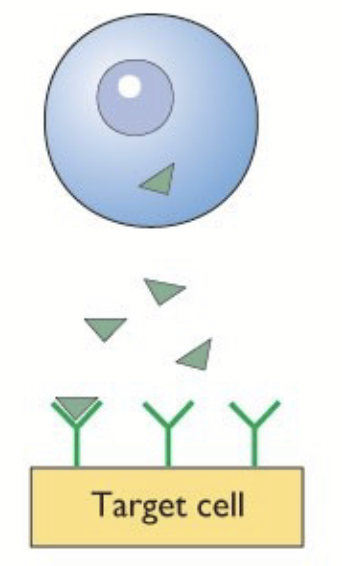
water-soluble hormones
Secreted (exocytosis). Circulate free in the blood. Stay out of target cell → surface (extracellular) receptor.
e.g. proteins and catecholamines.
carrier proteins
Big proteins. Bind to lipophilic proteins to help them circulate in blood. Keep hormones in vessel and prevent their degradation.
Hormone + Carrier ⇌ Hormone-Carrier (reversible binding)
Serves as “hormone reservoir” / “hormone buffer” / “hormone protection”.
Amount of these proteins is EQUALLY important as the production of the hormones!
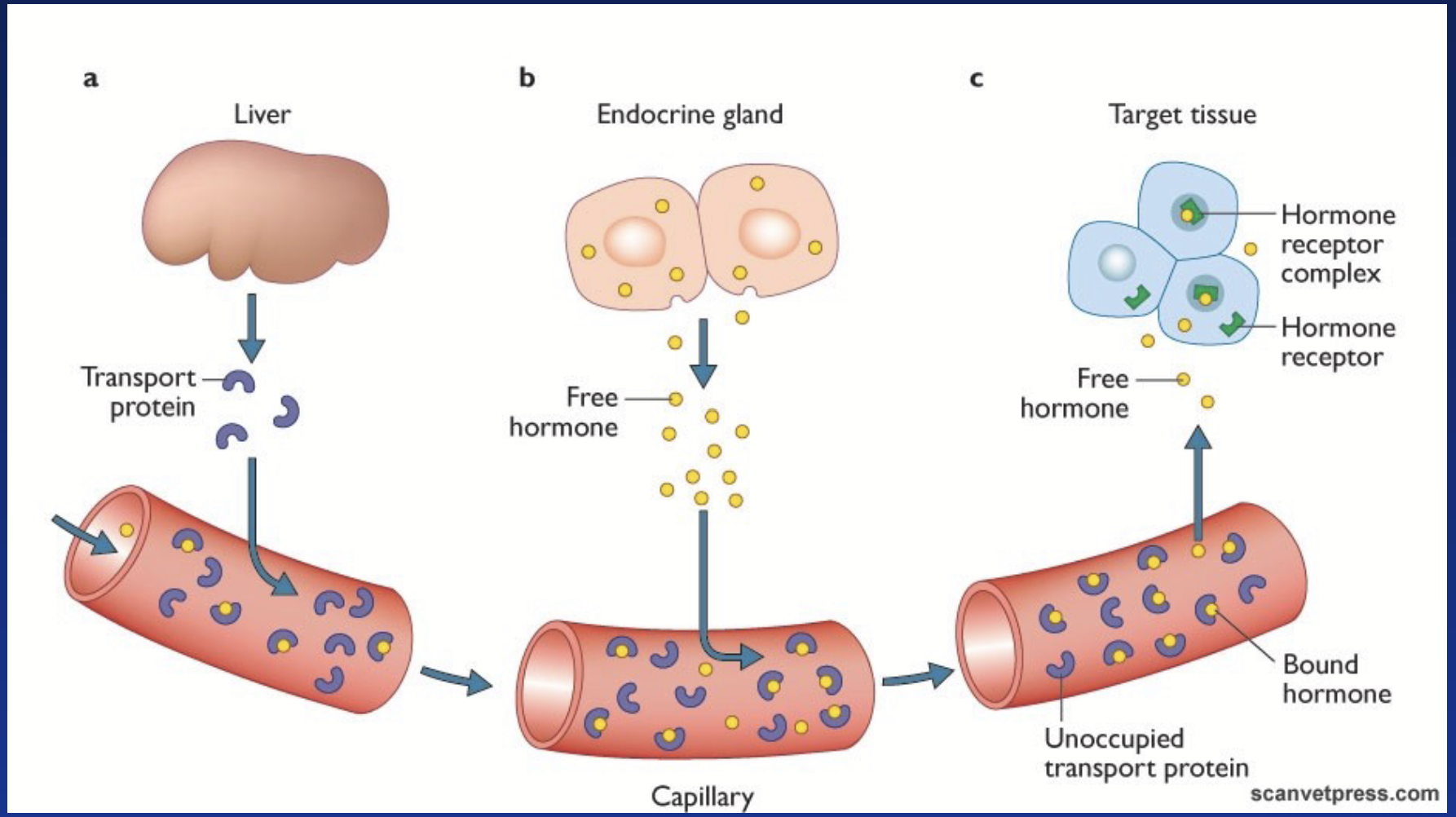
action of lipophilic hormones
Free hormones diffuse through plasma membrane of target cell. They bind to a specific intracellular receptor = nuclear hormone receptor (NHR). Hormone-receptor complex translocates to the nucleus and binds to specific DNA sequence (response element). Stimulates gene expression → de novo protein synthesis.
The action affects the synthesis of new proteins by gene regulation = slow-acting hormones.
After acting, hormones dissociate from the receptor, can be partly degraded in target cells (thyroid hormones), then go back into circulation and is degraded in the liver.
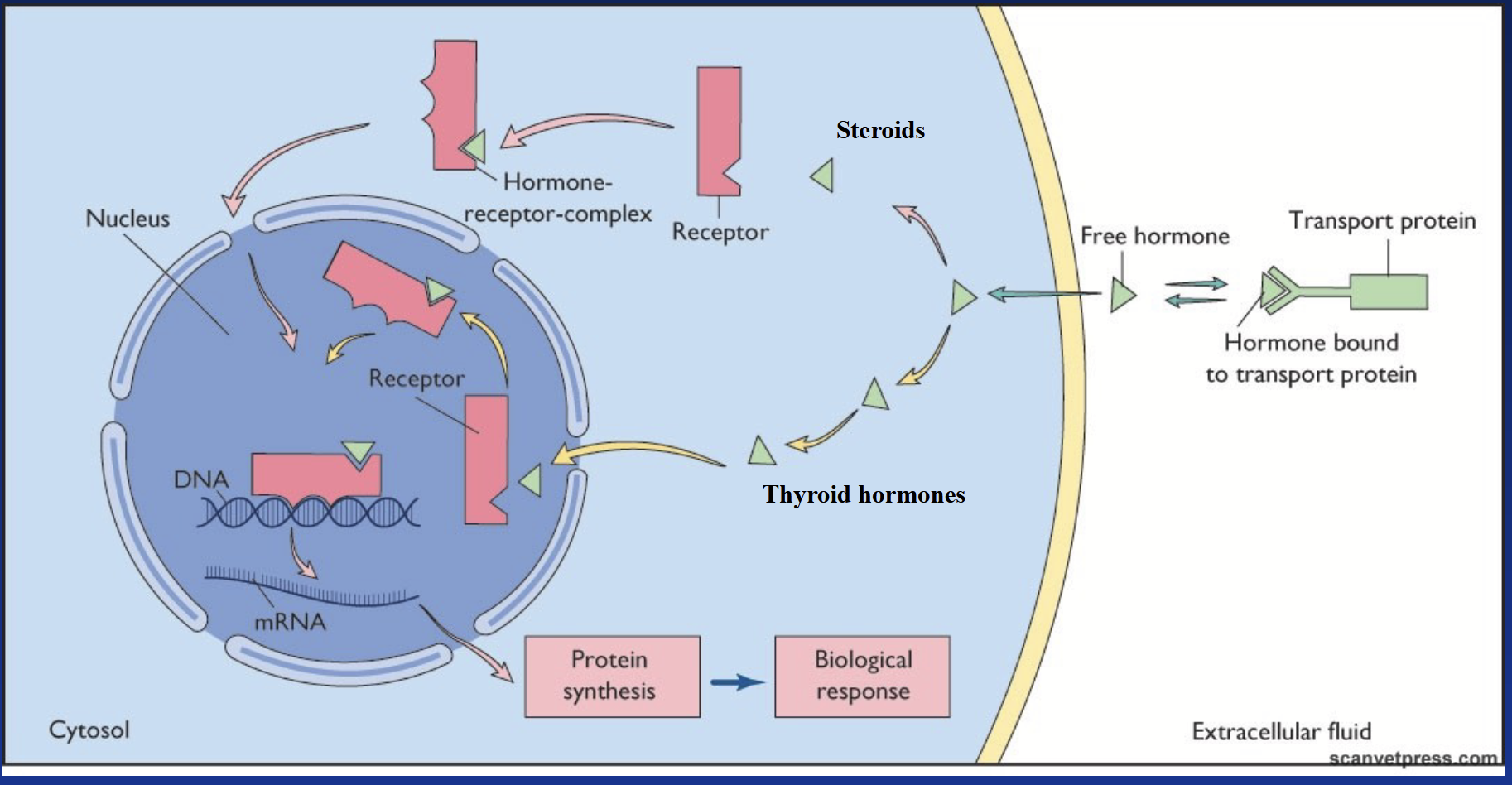
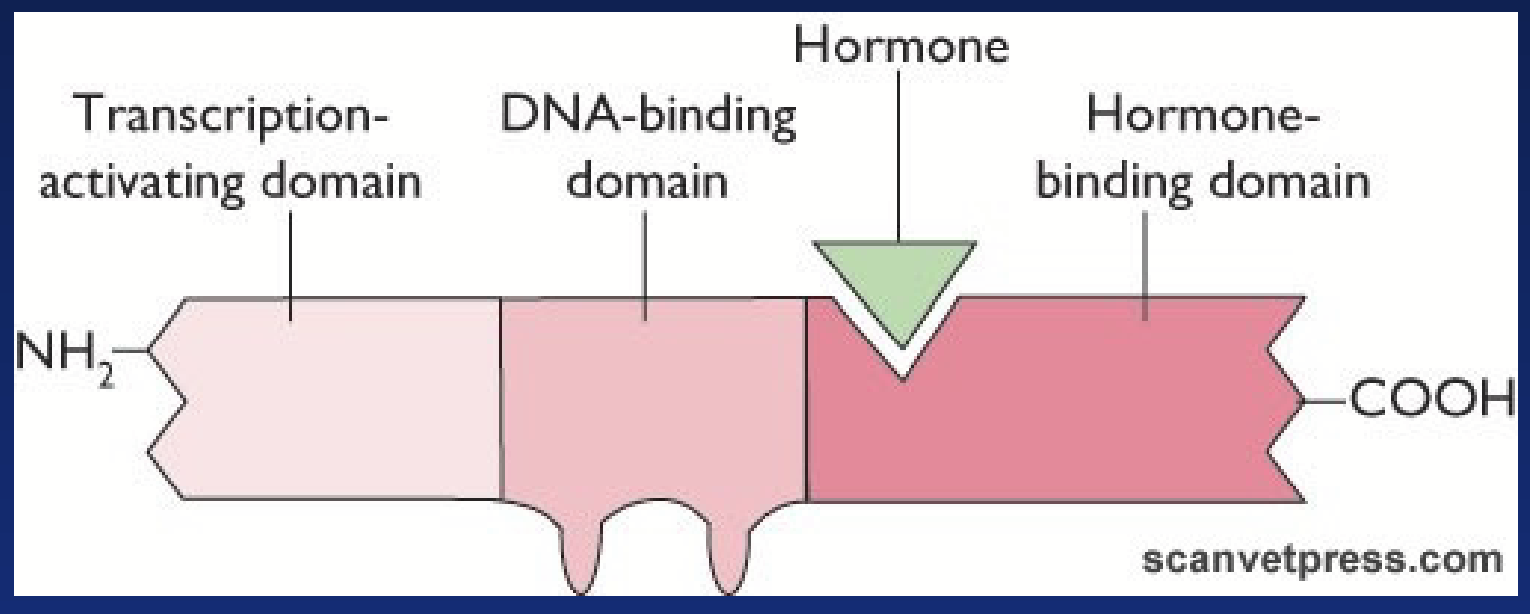
nuclear hormone receptor (NHC)
Specific intracellular receptor that binds lipophilic hormones. Regulates gene transcription - considered “transcription factors”.
In the case of thyroid hormones, the receptor is already in the nucleus and hormones diffuse all the way there.
Ligand binding domain: binds hormone.
DNA binding domain: binds DNA of target gene. Contains zinc fingers. Gives specificity of receptor.
Activation domain: stimulates gene transcription.
Orphan receptor: cloned but no ligand found (yet).
action of lipophilic hormones: thyroid hormones
Major circulating form is T4 (thyroxine). When it enters the target cell, it is converted to T3 (tri-iodo-thyronine), the cellular active form. Binds to its specific receptor after entering the nucleus.
action of water-soluble hormones
Cannot pass the phospholipid membrane barrier. Secreted in vesicles by exocytosis, circulate free (no carrier proteins needed to solubilize in blood).
EXCEPTION: IGF-1 circulates bound to carrier.
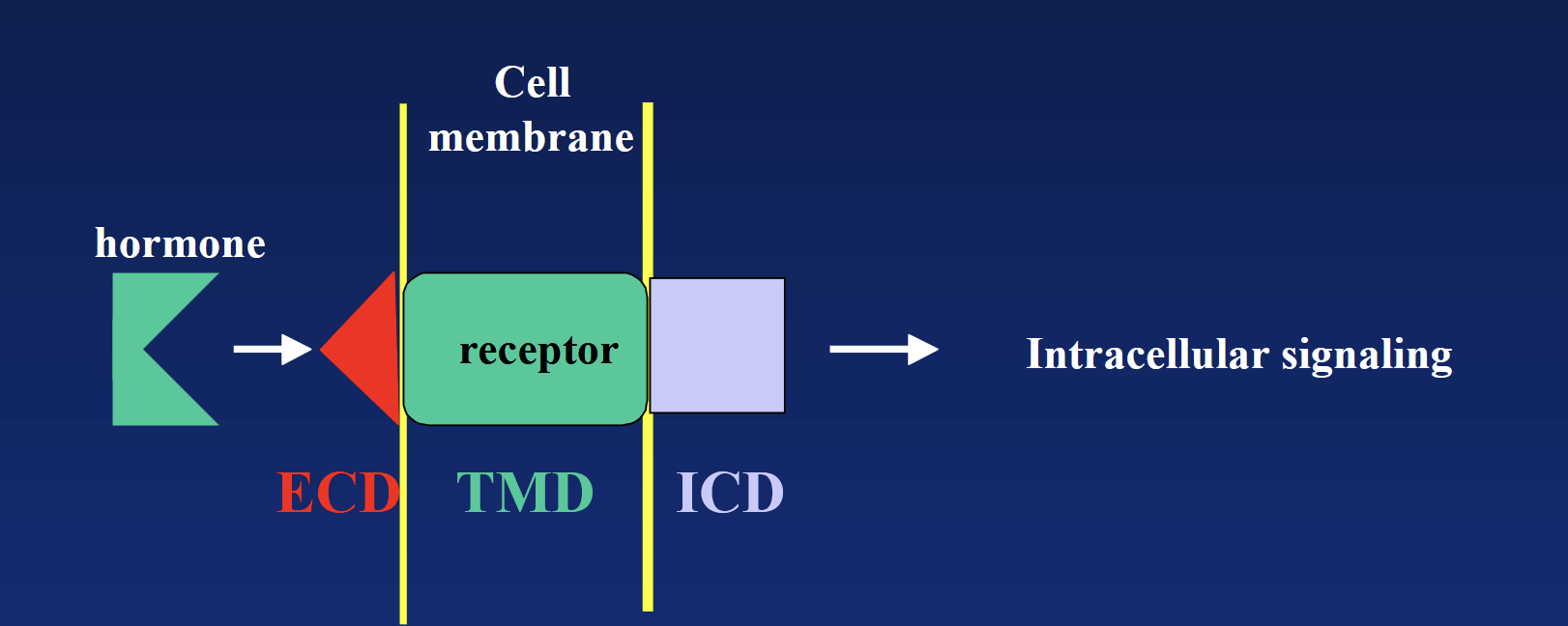
Bind to specific receptors on surface of target cell (mediate action via transmembrane receptors). DO NOT enter the cell to act - NEED SECOND MESSENGERS. Receptor is the key mediator between hormone and second messengers!
Hormone → ECD → TMD → ICD → second messenger cascade → intracellular signaling (usually enzymatic reaction).
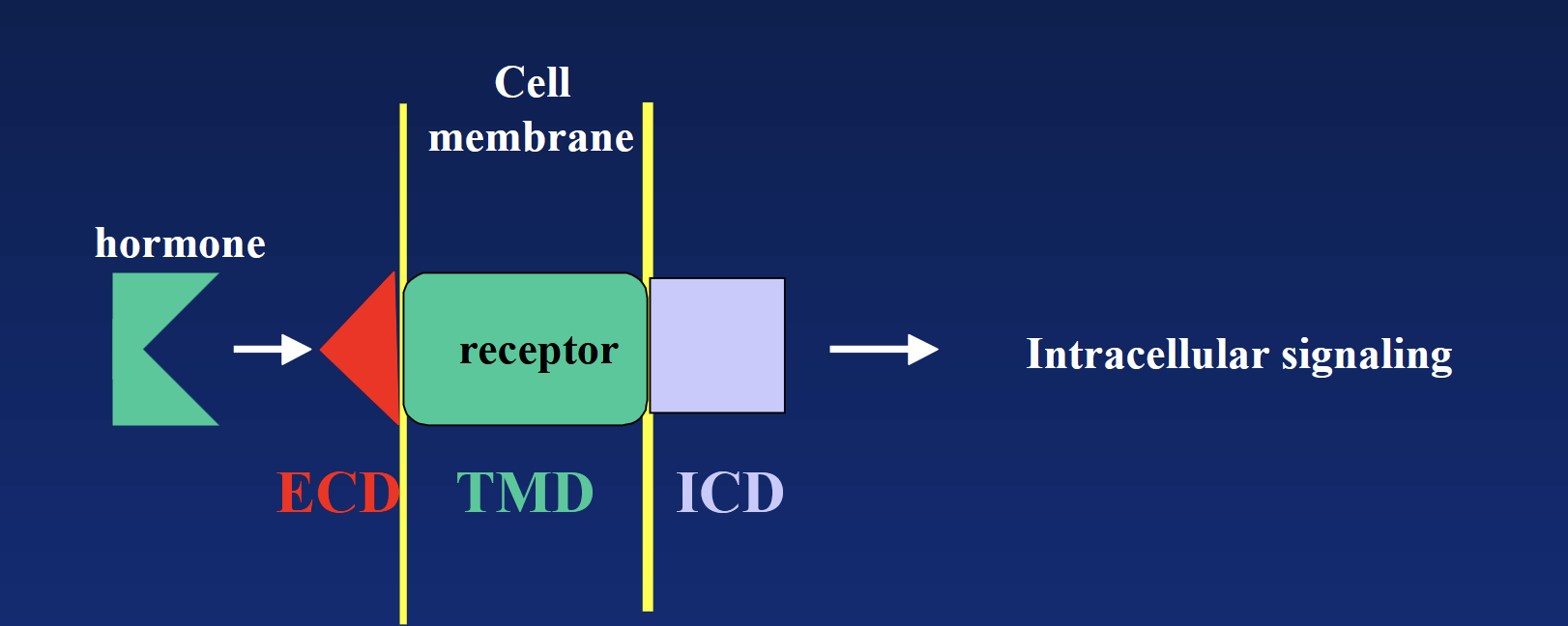
cell surface receptor
Transmembrane domain (TMD): hydrophobic region “hiding” in the membrane’s phospholipids.
Extracellular domain (ECD) + intracellular domain (ICD): hydrophilic regions located outside and inside.
Hormone-Receptor: activation of intracellular second messenger.
Hormone → ECD → TMD → ICD → second messenger cascade → intracellular signaling (usually enzymatic reaction).
second messengers
Messengers that amplify the hormone signal after water-soluble hormone binds to cell surface receptor. Results in intracellular signaling.
major types of cell surface receptors
G-protein coupled
Tyrosine kinase
Interleukin/cytokine family
Serine kinase (TGFβ) family
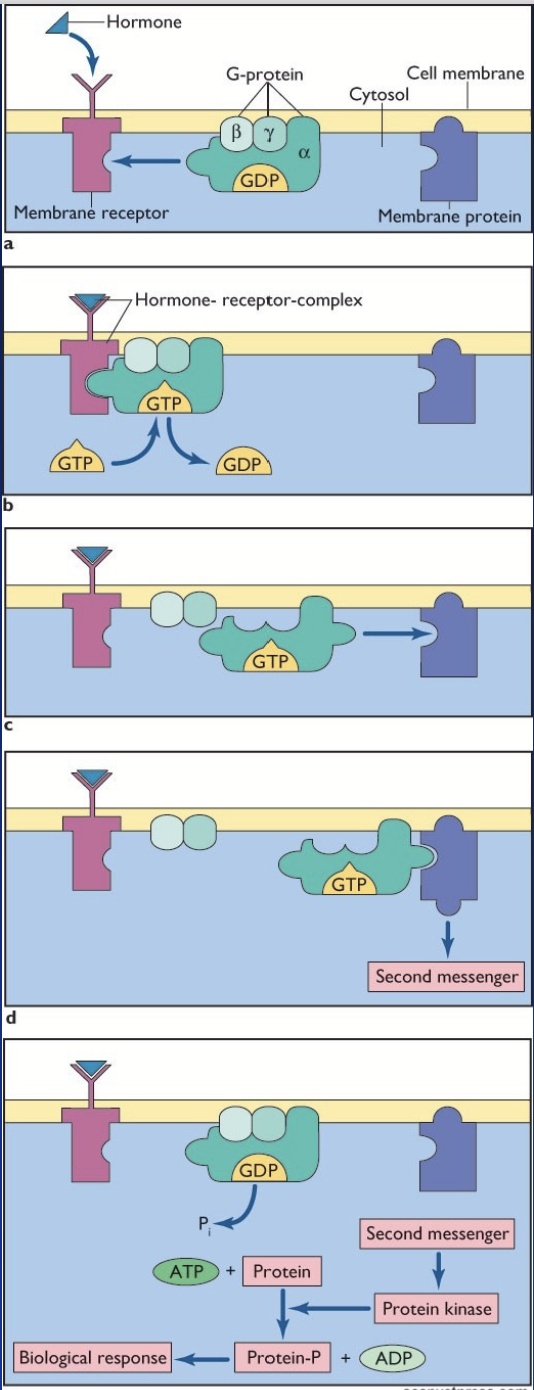
G-protein couple receptors
THREE major types differing by their α subunit.
Action:
Hormone binds to a receptor coupled to G-proteins (αβγ subunits).
Change in receptor conformation = exchange of GDP with GTP on Gα subunit.
Gα subunit dissociates from βγ subunits and activates a membrane protein.
Activated membrane protein stimulates a cascade of second messengers.
The second messengers elicit the biological response in the cell.
adenylate cyclase-cAMP-PKA pathway
cAMP-dependent pathway!
Receptor coupled to G-proteins αs or αi (β-adrenergic, LH).
Membrane-associated enzyme is adenylate cyclase (AC).
Gαs stimulates AC (increase cAMP); Gαi inhibits AC (decreases cAMP).
AC hydrolyzes ATP into cyclic AMP (cAMP).
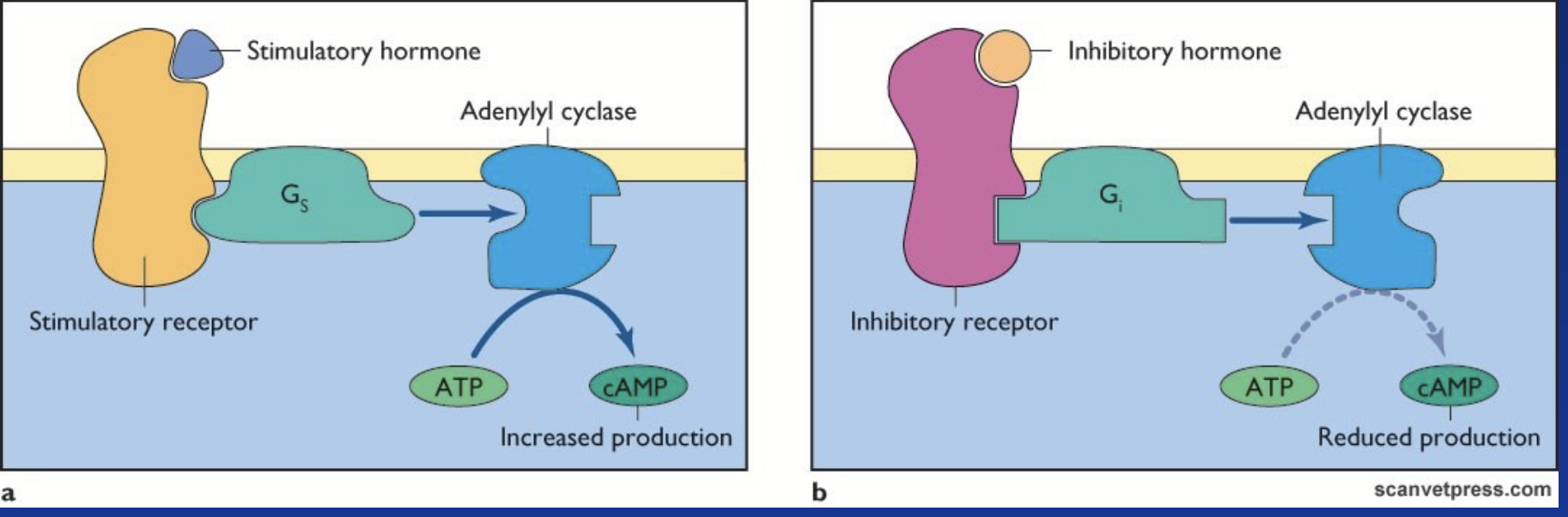
adenylate cyclase (AC)
Membrane-associated enzyme in the adenylate cyclase-cAMP-PKA pathway.
Stimulated by G-protein Gαs. Inhibited by G-protein Gαi.
Hydrolyzes ATP into cyclic AMP (cAMP).
phospholipase C pathway (PLC)
Ca2+-dependent signaling!
Receptor coupled to G-protein αq. Membrane-associated protein is phospholipase C (PLC). Gαq stimulates PLC.
Results in the activation of:
Ca2+/calmodulin = release of Ca2+.
Protein kinase C = phosphorylation cascade.
Biological action.
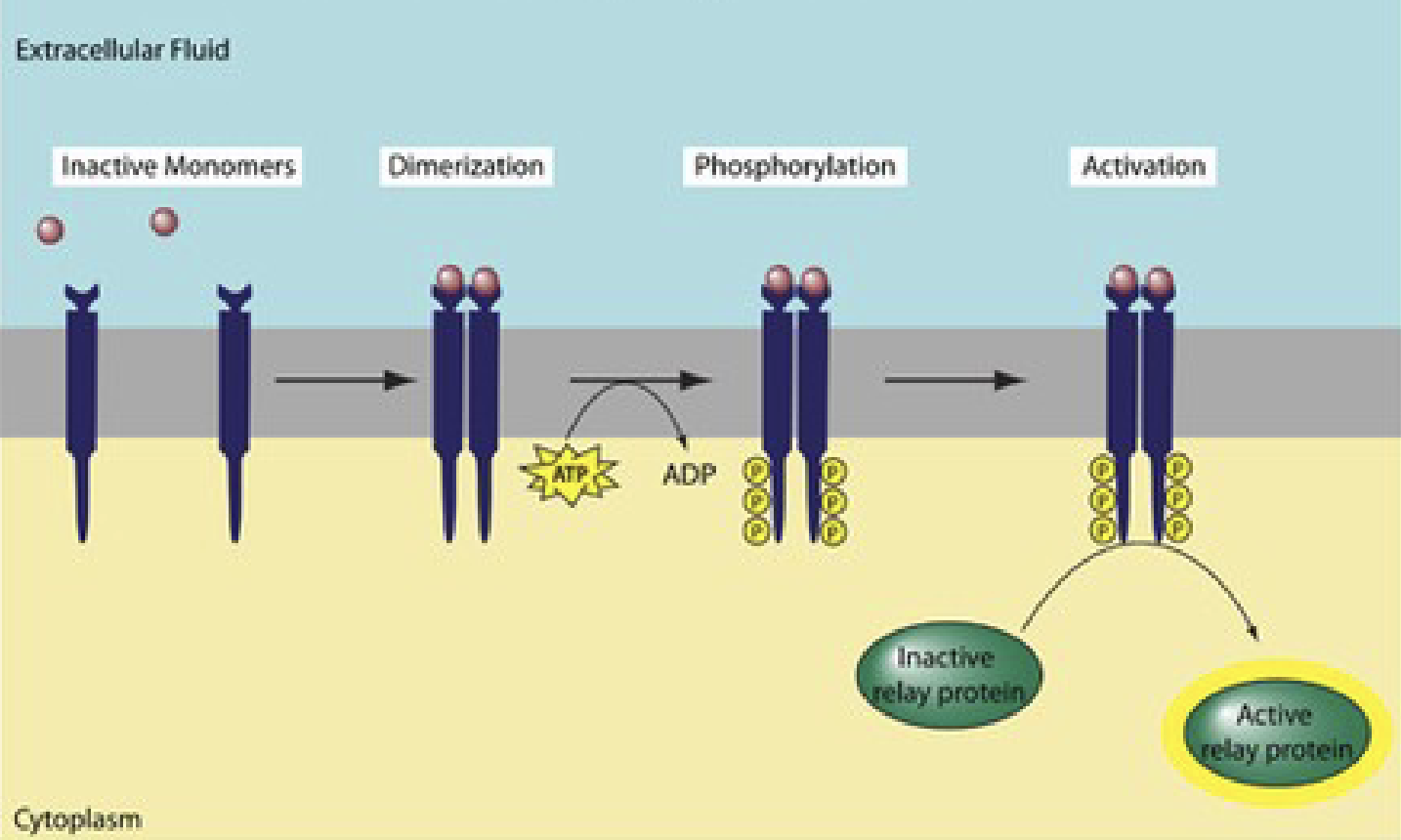
tyrosine kinase receptor
Binding of hormone → activation of receptor = autophosphorylation → becomes a kinase → phosphorylates tyrosines on target proteins.
Receptor used by insulin!
No need for second messenger like G-coupled proteins, instead, directly phosphorylates target proteins.
Consists of three domains:
Transmembrane domain
Extracellular domain for ligand recognition
Cytoplasmic domain with autophosphorylation site that transmits regulatory signals and contains ATP binding sites
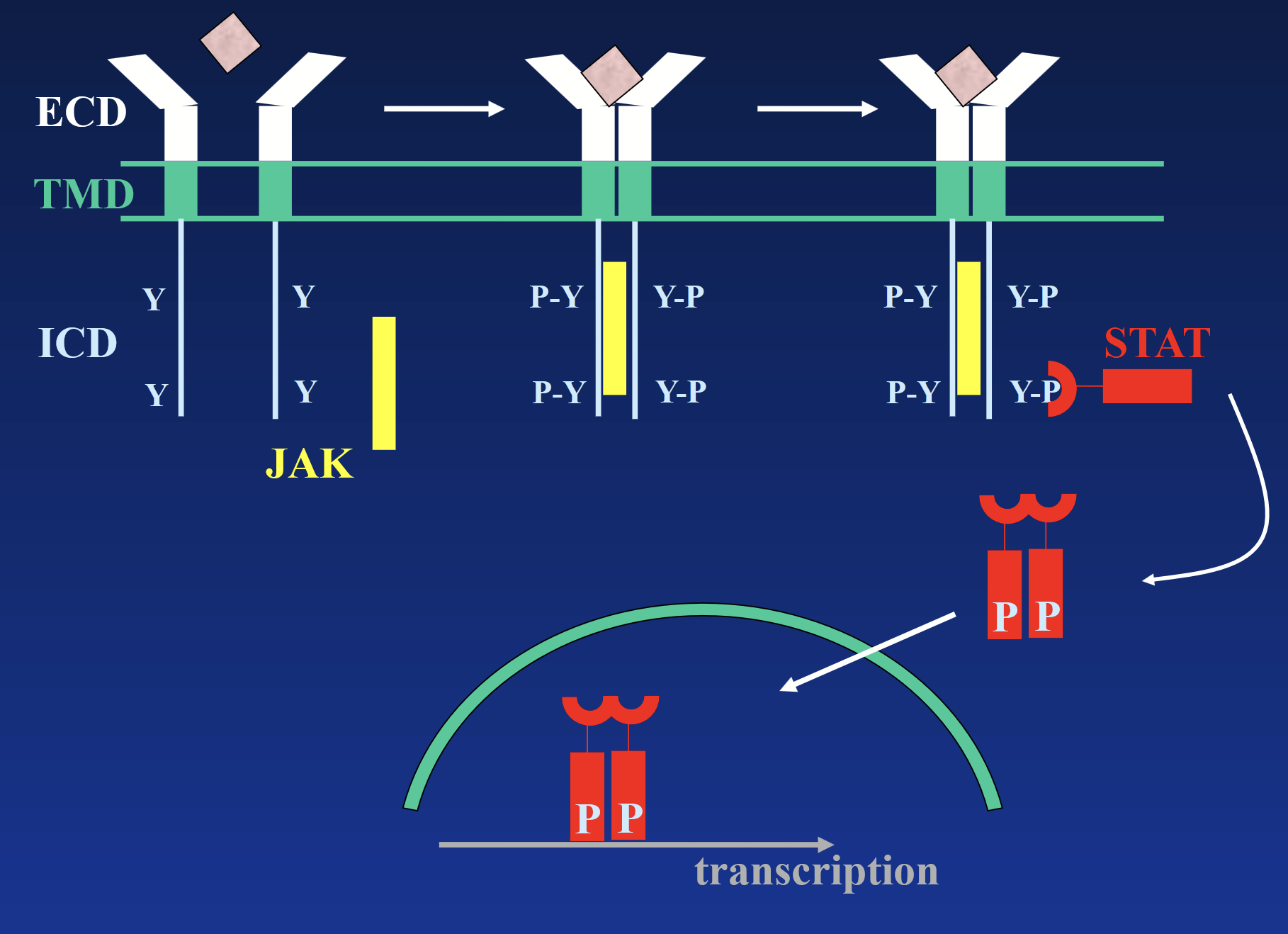
interleukin/cytokine receptors
These receptors DO NOT have intrinsic kinase activity. Receptor exists as a monomer. Binding of hormone causes dimerization and binding of JAK tyrosine kinase, which phosphorylates the receptor.
Phosphotyrosines act as docking sites for intracellular signaling molecules - STATs (signal transducers and activators of transcription), which activate various genes.
STATs trigger second messengers → signal cascade → transcription.
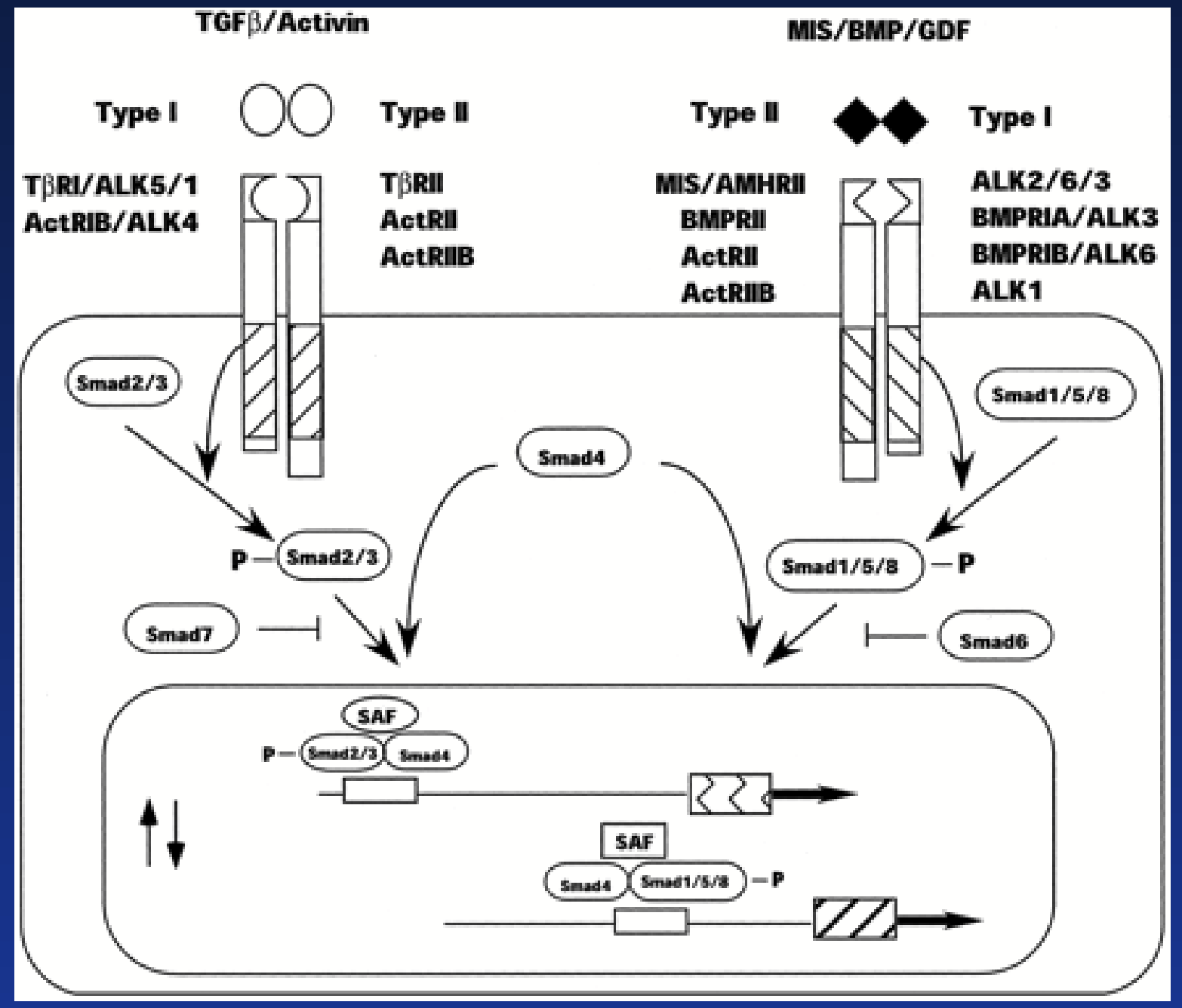
receptor serine kinase
TGFβ family (activin, inhibin, MIS) mainly involved in control of cell proliferation and differentiation.
Binding of hormone results in heterodimer formation: Receptor I + Receptor II (not identical).
RII is specific to the hormone. After binding, H-RII complex recruits RI. Same RI can be recruited by different H-RII complexes!
Serine residues on RI get phosphorylated by RII.
Activated receptor phosphorylates SMADs proteins that will dimerize, translocate in the nucleus, and modulate gene transcription.
action of cell surface receptors
Cascade of intracellular messengers amplifies the signal several thousand times.
Specific effects on target cells depend on the type and amount of messenger activated:
Immediate effects: enzyme activation (usually multiple), exocytosis.
Slow effect: stimulation of gene transcription, de novo protein synthesis.
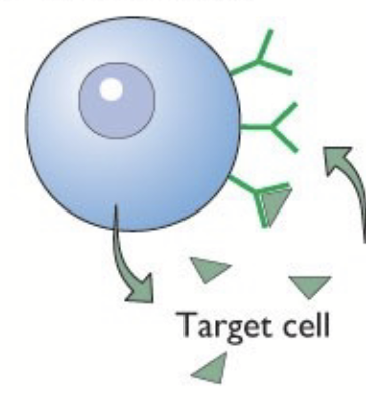
After signalling, receptor-hormone complex is internalized (signal termination):
Fuses to lysosome and is degraded.
Dissociates and receptor can be recycled to the cell surface.
Once receptor is inside cell, cannot bind hormones - if all receptors are in cell, cannot bind any hormone → desensitization.
desensitization
Once receptor is inside cell, cannot bind hormones - if all receptors are in cell, cannot bind any hormone.
hypothalamo-pituitary axis
Hypothalamus composed of neuroendocrine cells. Some project axons down the posterior pituitary lobe. Some release factors into the pituitary stalk portal venous system to feed the anterior pituitary.
If released into general circulation: concentration too low, would be quickly deactivated.
Endocrine cells from the anterior and intermediate pituitary release their hormones in a second capillary network → enter systemic circulation.
hypothalamus
The major integration center. Composed of neuroendocrine cells. Part of the brain, regulates the autonomic nervous system, regulates most of the endocrine system. Processes most sensory information.
pituitary gland
Small gland attached to the hypothalamus.
Three parts: posterior, intermediate, and anterior.
posterior pituitary lobe
“Neural” part of the pituitary = neurons from hypothalamus released hormones into blood.
anterior pituitary lobe
“Master gland” = major endocrine part of pituitary (glandular tissue), produces hormones.
intermediate pituitary lobe
Major function in amphibians and fish (MSH). Potential source of β-endorphins.
anterior pituitary
Often considered as anteior + intermediate lobes. Releases prolactin, LH, FSH, GH, TSH, and ACTH.
portal venous system
Some neuroendocrine cells release factors into a system to feed the anterior pituitary. Factors do not enter general circulation (would be too low concentration and quickly degraded).
Capillary bed is connected to another capillary bed → door to door delivery → bypass general circulation.
melanocyte stimulating hormone (MSH)
Hormone produced by the intermediate pituitary. Increases skin pigment Major function in amphibians and fish.
Derived from common gene POMC.
β-lipotropin (β-LSH)
Hormone released by intermediate pituitary. Degraded to β-endorphin = analgesia during stress (body’s anesthetic).
Derived from common gene POMC.
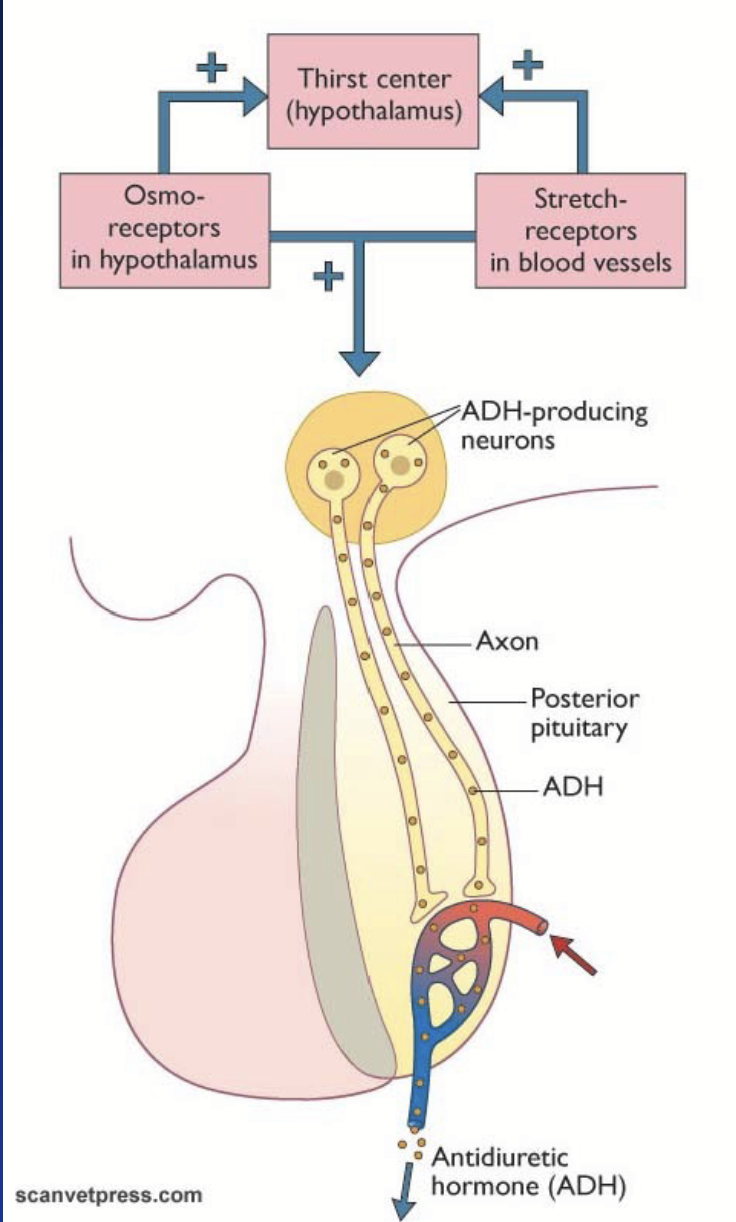
posterior (nervous) pituitary
Releases antidiuretic hormone (ADH/vasopressin) and oxytocin produced in the cell body of hypothalamic neurons.
Transported to the pituitary along axons in vesicles. Stored in nerve endings, released when AP is fired. After secretion, hormones diffuse into blood vessels.
anti-diuretic hormone (ADH)
Most important regulator of extracellular fluid.
Controls water intake + water balance at level of kidneys.
Acts in the kidneys: regulates the density of aquaporins (water channels) in the distal tubule and collecting duct.
Increases reabsorption of water.
Primarily regulated by hypothalamic osmoreceptors and stretch receptors in blood vessels.
Reflex in nervous system - axons release hormone in blood, then carried through body.
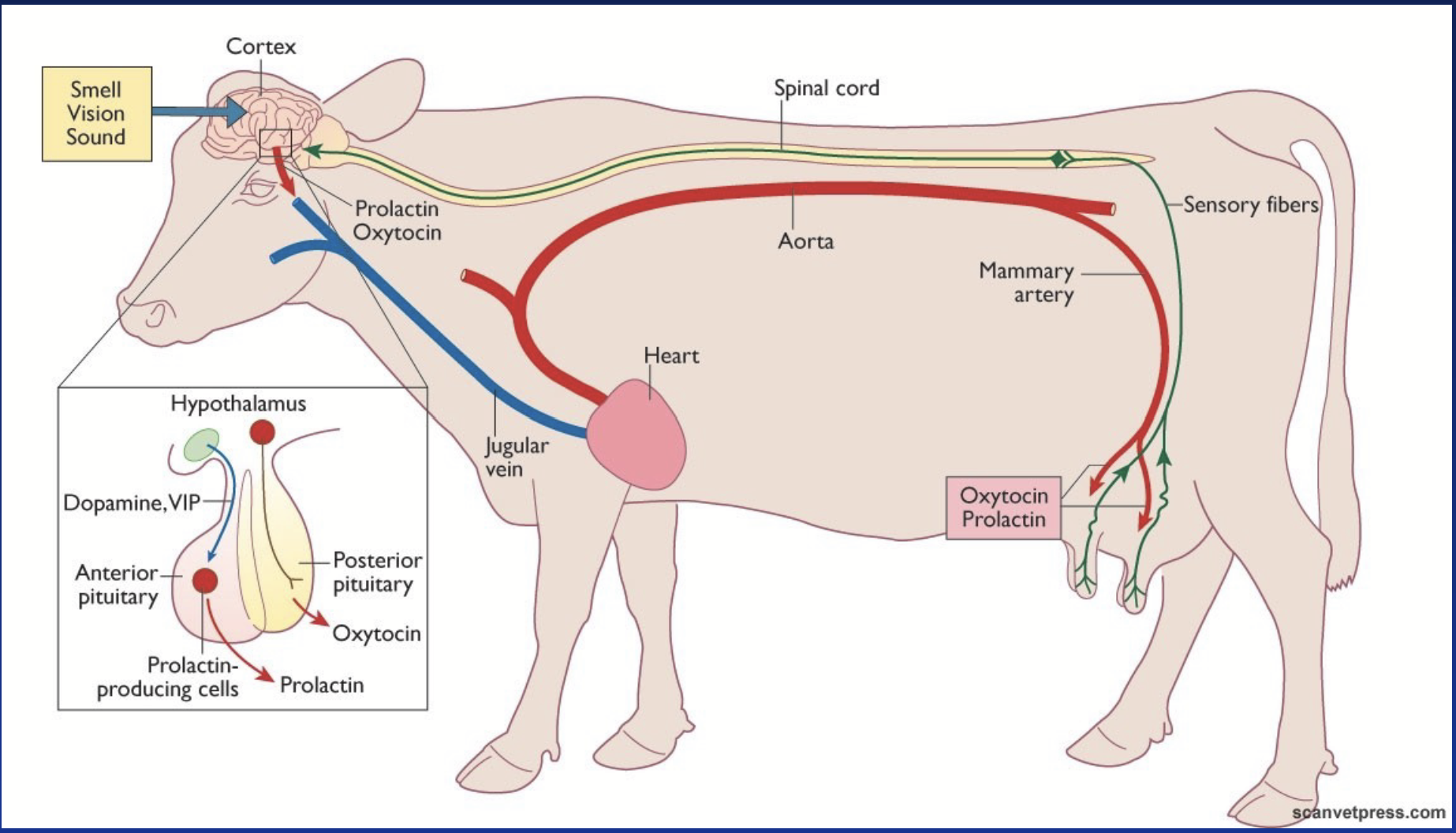
oxytocin
Hormone produced in the posterior pituitary.
Primarily acts on:
Uterus smooth muscle - contraction during parturition.
Mammary gland - contraction increases pressure to drive milk towards excretory ducts and the teats (milk ejection reflex).
Receptor = G-coupled receptor with activation of PLC (Ca2+ pathway).
Secretion regulated by several reflexes.
milk ejection reflex
Oxytocin can be used to induce milk ejection during first partuition. Cow may become dependent on external oxytoxin and may become desensitized.
Seeing and hearing calf enhances the response.
anterior pituitary (master gland)
Endocrine part: contains 5 different cells producing 6 different hormones - proteins or glycoproteins with longer half-lives than their releasing hormones.
TSH → thyrotrope → targets thyroid gland
LH & FSH → gonadotrope → targets gonads
ACTH → corticotrope → stimulates adrenal glands
GH → somatotrope → growth (immature) + metabolism (mature)
PRL (prolactin) → mammotrope (lactotrope)
Tropic effects = regulate other endocrine glands.
Under direct control (positive and/or negative) from hypothalamus.
growth hormone (GH; somatotropin)
Cytokine receptor type. Originates from cells of the anterior pituitary, from somatotrope cells.
Promotes tissue and body growth in young animals, especially longitudinal growth (skeleton) until skeleton is complete. In adult, mainly controls metabolism.
Protein sequence differs a lot between species (poor cross-reactivity).
Direct effects: stimulates lipolysis (increases fatty acid production) and reduces lipogenesis in adipose tissue (catabolic); promotes synthesis of protein (anabolic); decreases glucose utilization in most tissues (blood glucose increases).
Indirect effects: GROWTH by stimulating synthesis of IGF1 (somatomedin) and its binding proteins in the liver. Stimulates chondrocyte (cartilage cells) proliferation to increase bone growth; stimulates satellite cells in muscle (muscle fibre growth); stimulates amino acid uptake and protein synthesis.
insulin-like growth factor 1 (IGF1)
Polypeptide chain with good cross-reactivity. Synthesis (along with its binding proteins) in the liver stimulated by GH. Also locally produced in muscle + adipose tissue. Affects growth.
Bound to carrier proteins. Half-life is much longer than GH. Acts via tyrosine kinase receptors.
Stimulates chondrocyte (cartilage cells) proliferation to increase bone growth; stimulates satellite cells in muscle (muscle fibre growth); stimulates amino acid uptake and protein synthesis.
Stimulates growth during fetal development along with IGF2.
thyroid-stimulating hormone (TSH; thyrotropic hormone)
Gαs receptor type!
Binds to its G-coupled receptor on membrane of follicular cells in thyroid gland, stimulates cAMP pathway which in turn stimulates the synthesis of thyroid hormones.
adrenocorticotropic hormone (ACTH)
Receptor is a Gαs-coupled receptor stimulating the cAMP pathway.
Stimulates the mobilization of cholesterol in adrenal cortex = more substrate for cytochrome P-450 → increases release of corticosteroids.
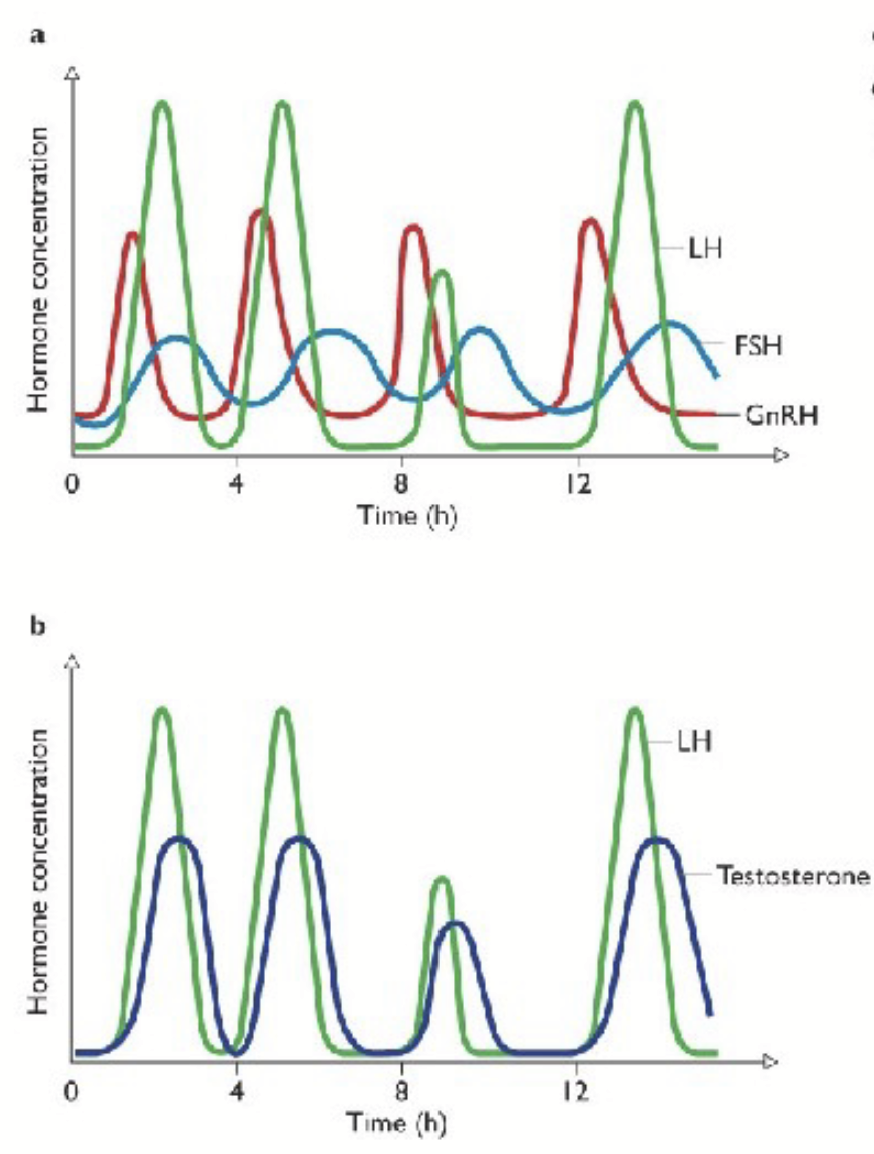
lutenizing hormone (LH)
Gαs-protein coupled receptor (cAMP pathway).
In male: stimulates testosterone production by Leydig cells in the testes.
In female: controls sex steroid production by the ovary and is responsible for ovulation (surge).
follicle-stimulating hormone (FSH)
Gαs-protein couple receptor (cAMP pathway).
In male: stimulates secretion of inhibin by Sertoli cells.
In female: development of follicles and secretion of sex steroids.
prolactin (PRL)
Cytokine receptor type (same ancestral gene as GH, so similar mechanism).
Stimulates the synthesis of milk proteins (casein, lactalbumin).
In poultry, responsible for the initiation and maintenance of incubation behaviour (broodiness).
Under tonic control (tonic inhibition) - always turned off by default!
In mammals: under dopaminergic tonic inhibition. In birds: mainly under VIP stimulation.
hypothalamic control of anterior pituitary
Neurohormones released in small amounts (bypass general circulation) into the portal venous system.
GnRH and GnIH
ACTH-RH
TRH
GH-RH and GH-IH
Dopamine
VIP in birds
Hypothalamic neurons receive information from:
Higher brain center, emotions (integrates with consciousness - cerebrum).
Exterior, environmental, and social stimuli.
Internal rhythms.
Metabolic state (temperature, energy level, osmolarity).
Endogenous hormones by feedback.
tonic inhibition
Hormones are turned off by default - e.g. GH, MSH, prolactin.
These hormones are needed early in life. As hypothalamus matures and animal ages, secretion decreases.
pulsatility
Many hormones from the hypothalamus and pituitary are pulsatile or episodic → regulated by the biological clock of hypothalamic suprachiasmic nucleus. May prevent the down regulation of receptors from continuous level of hormone secretion. Can trigger specific action depending on pulse frequency.
Release is NOT continuous from hypothalamus/pituitary → prevents desensitization (receptors do not become overwhelmed).
short feedback loop
Pituitary hormones feed back to hypothalamus.
long feedback loop
Hormones from target glands feed back to pituitary and hypothalamus. Most prominent.
GH secretion
Regulated by hypothalamic neurohormones: GHRH and GHIH → very precide dual regulation!
Hypothalamus receives info from external stimuli and nutrients in the blood:
Follows circadian pattern during sleep, increased during sleep.
High amino acid content and low glucose content in plasma both increase secretion (high protein diet, long fasting) → triggers energy release and protein synthesis.
Corresponds with sleeping because blood sugar decreases during sleep.
Exercising and stress increase secretion (greater energy need).
Sex steroids increase secretion → burst of growth at puberty.
IGF1 responsible for negative feedback on secretion.
GH and lactation
No major role in mammary glad development, Effects on milk production are mainly mediated via metabolism and partitioning nutrients to the mammary gland for milk synthesis.
Insulin normally directs nutrient towards lipogenesis in adipose tissue. GH has opposite effect.
Bovine GH (bST) can boost milk production by 10 and 40% in early and late lactation, respectively.
Major concern for humans: infant can absorb GH and IGF in the gut (IGF1 has similar sequence in bovine and human, whereas GH has low cross-reactivity) → infant GIT not fully developed, can absorb full proteins.
overproduction of Gh
Rare in animals.
Before puberty (before skeleton formed): gigantism (increased longitudinal growth).
After puberty (no more longitudinal growth): acromegaly (increased flat bone density), increased bone width and density, diabetes.
In old dogs and cats: can be due to pituitary tumour (negative feedback does not work properly).
lack of GH production (or problem with GH receptor)
Results in dwarfism.
Mostly genetic defects of GH or GH receptor genes, can be treated by inject of recombinant GH and IGF1 (synthetic).
In dogs, dwarfs keep their puppy coat for a long time due to lack of IGF1 action on hair follicle differentiation.
pancreas
Endocrine and exocrine gland.
Endocrine = islets of Langerhands (scattered throughout) → β cells (70%)n secrete insulin, α cells (20%) secrete glucagon.
insulin and glucagon
Protein hormones secreted by the pancreas. Have opposite effects on glucose metabolism.
insulin production
Produced as a preprohormone, converted to proinsulin.
Before secretion, converted to active form by removing a connecting (C-) peptide.
After secretion, circulates free (half-life of 5-8 min) and is degraded in target cells or in liver.
glucagon production
Produced as a preprohormone rapidly converted to active form.
After secretion, circulates freely (half-life of 5 min), and is metabolized in liver and kidney.
insulin
~20 AA peptide. MAJOR anabolic hormone of the body. Through its tyrosine kinase receptor, insulin leads to the upregulation of membrane glucose transports → CELLULAR UPTAKE OF GLUCOSE in muscles and adipose tissue.
Stimulates incorporation of glucose in energy storage molecules: glycogen in liver + muscle, and triglycerides in adipose tissue.
Also promotes uptake of amino acids.
Overall effects lead to a DECREASES IN BLOOD GLUCOSE AND AMINO ACID LEVELS.
glucagon
29 AA long peptide. Has opposite effect to insulin. Through its G-protein coupled receptor, activates enzyme responsible for glycogenolysis → release of glucose from glycogen by the liver.
Also stimulates gluconeogenesis in the liver (synthesis of glucose from scratch).
Stimulates release of fatty acids from triglycerides in adipose tissue.
regulation of insulin and glucagon secretion
Major mechanism = blood glucose concentration and, to a lesser extent, amino acid concentration.
High blood glucose = increased insulin → glucose uptake into cells.
Low blood glucose = increased glucagon → release of glucose and FFAs.
Autonomic nervous system: during meals, parasympathetic activates insulin secretion. Sympathetic inhibits insulin secretion and stimulates glucagon release = “stress hyperglycemia”.
diabetes mellitus
Hyperglycemia. When blood glucose levels exceed kidney reabsorption capacity → glycosuria → increase osmolarity of urine → increase in urine volume (diabetes), sweet (mellitus).
Insulin-dependent: insulin secretion is impaired = Type I (β-cells destroyed or damaged, usually via autoimmune disorder, or defective insulin/receptor).
Insulin-independent: insulin secretion is normal but tissues are insensitive = Type II.
For humans, insulin supplementation will not work because the problem is usually with the receptors.
In animals, is only observed in cats and dogs, mainly due to inflammation of pancreas - treat animal with exogenous insulin.
thyroid gland
Located on both sides of the trachea, below the larynx. Two lobes bridged by isthmus. Heavily vascularised (fed straight from carotid artery, direct blood supply) - heaviest of pure endocrine glands.
Composed of numerous follicles:
Single layer of epithelial cells that release thyroid hormones.
Lumen filled with protein rich fluid (colloid) → where iodine goes to produce thyroid hormones.
Between follicles, parafollicular cells (C-cells) - secrete calcitonin.
synthesis of thyroid hormones
Active uptake of iodine: iodine circulates in blood as iodide, actively transported through follicular cells (I/Na symport), diffuses and accumulates in colloid as iodine (I2).
Iodination of thyroglobulin (Tgb): Tgb, a glycoprotein with over 120 Tyr residues, is released in the lumen. Tyrosine peroxidase (TPO) binds I2 to Tyr residues. In colloid, 10% of Tgb is iodinated. Each Tyr residue can be iodinated on possibly two sites:
One I2 Tyr = monoiodotyrosine
Two I2 tyrosine = diiodotyrosine
Tgb-I2 taken by endocytosis from colloid incells, fused to lysosomes and split into thyroid hormones (chop every two Tyr residues).
tetraiodothyronine/thyroxine (T4)
Made of two diiodothyrosines. Inactive form of thyroid hormone. Represents 90% of thyroid hormones.
triiodothyronine (T3)
One monoiodotyrosine + one diiodotyrosine. Active form of thyroid hormone, has high affinity for receptors Makes up 10% of thyroid hormones.
transport of thyroid hormone
Thyroid hormone binding globulin (TBG) binds 70-80% of circulating thyroid hormones. Remaining is bound to other albumins.
Only 0.03% of T3 and T4 are free in blood and diffuse to tissues (only free hormone is active). Thus, there is several days’ worth of hormone available bound to TBG.
Free and bound forms are in equilibrium - reversible!
thyroid hormone action
After diffusing in target cells, T4 is deiodinated to T3. T3 binds to its nuclear receptor and activates gene transcription. Affinity of receptor is higher for T3 than T4 → T3 is five times more active.
Thyroid hormones act on all cells of the body to increase basal metabolic rate → increase heat production (calorigenic), O2 consumption (except brain, gonads, and spleen).
During development, thyroid hormones stimulate secretion of GH → important for bone growth and tissue growth (deficiency can lead to dwarfism).
During last phase of fetal development and early stage after birth, thyroid hormones are critical for normal maturation of brain function (lack leads to cretinism).
Thyroid hormones increase number of adrenaline/noradrenaline receptors → boost sensitivity to sympathetic system.
Essential for gonadal normal function (lack results in reduced sperm production and irregular cycles → infertility).
Increase nerve conduction velocity (faster reflexes).
thyroid hormone metabolism
Deiodination to reverse T3 in target tissues results in deactivation (now unable to bind receptor).
After deactivation, diffuses out of target cell and is processed in the liver - conjugated to other compounds to make them water-soluble, can then be excreted.
The I2 release during metabolism can be recycled to the thyroid (re-enter bloodstream and reused by gland to produce thyroid hormones) → iodine levels are very inconsistent in the body - cannot rely on what’s in blood, therefore, rely on active transport of I via pump.
regulation of thyroid hormone
TRH from hypothalamus increases TSH secretion from anterior pituitary.
TSH binds to its Gαs-coupled receptor on membrane of follicular cells, stimulates cAMP pathway which in turn stimulates the endocytosis of Tgb-I2 and synthesis of TH.
TH exert negative feedback on both pituitary and hypothalamus.
Exposure to long-term cold = increased TRH release (stimulates BMR to produce heat).
If metabolites decrease (fasting), then T3 and T4 rapidly decrease.
If low blood glucose, TH stimulated (initiate metabolism to increase glucose).
hypothyroidism
In humans, mainly associated with iodine deficiency. Results in reduced growth (dwarfism) and mental issues - cognitive decline (associated with decreased fetal growth).
In dogs, most cases result from autoimmune disorders that destroy follicular cells = primary hypothyroidism (lab tests show low T4 and T3 with high TSH).
Secondary hypothyroidism = low secretion of TSH, mainly due to injury to pituitary or hypothalamus.
Most cells types of affected:
Reduced heat production and tolerance to cold (cannot raise BMR).
Sluggish animal, not feeding but gaining weight (because TH increases nerve velocity),
Impaired reproduction, constipation…
Treatment: T4 for the rest of life (hormone therapy).
goiter
Enlarged thyroid gland. Several causes: deficiency in iodine (another form of hypothyroidism), tumor and inflammation. Lack of thyroid hormones increase TRH and TSH release (lack of feedback due to lack of TH being made, stimulating multiplication (hyperplasia) and volume (hypertrophy) of follicular cells.
In coastal area, the air, vegetation, and marine organisms are a good source of iodine - inland iodine deficiency is more frequent in animals.
hyperthyroidism
Over-production of thyroid hormones. Frequent in cats, cause unknown!
Many target organs: big appetite associated with weight loss, excitable and nervous; increased heart rate, respiration, and digestion.
Treatment: surgery, radioactive I2 (accumulates in follicular cells and will destroy them from within) to destroy follicular cells.
adrenal glands
Paired organs capping the kidneys like a little hat. Consist of two layers: outer cortex (mesoderm-derived) and inner medulla (derived from neural crest).
Medulla is part of the ANS - equivalent to a ganglion, but with postganglionic cells secreting catecholamines (epinephrine, norepinephine) that diffuse in the blood.
adrenal cortex
Composed of three zones:
Zona glomerulosa → secretes mineralcorticoids.
Zone fasciculata → secretes glucocorticoids.
Zone reticularies → secretes androgens.
Makes over 90% of adrenal glands’ mass. Produces steroid hormones (adrenocortical hormones) required for survival.
Hormones produced by stepwise conversion of cholesterol previously absorbed from blood lipoproteins. Cytochrome P-450 = major enzyme in conversion. Each zone has different enzymes → formation of different hormones.
mineralcorticoids
Produced in the zona glomerulosa. Most potent is aldosterone. After diffucion, circulate loosely bound to cortisol binding clobulin (CBG).
Synthesis and secretion stimulated by angiotensin, and by direct action of K+ concentration on cortical cells.
ACTH from pituitary has minimal impact on aldosterone production.
Regulate metabolism of important inorganic ions → stimulate reabsorption of Na+ and the secretion of K+ in the distal kidney tubules and collecting ducts.
In target cells, aldosterone binds to its receptor to modulate gene transcription → increase in Na+-K+ pumps in basolateral membrane, and increase in Na+ channels in apical membrane.
angiotensin
Stimulate mineralcorticoid synthesis and secretion.
Released when there is a change in blood volume and water must be reabsorbed.
glucocorticoids
Hormones released from the adrenal cortex (zona fasciculata) that regulate glucose metablolism. Cortisol is the most potent, corticosterone has weaker activity.
After diffusing into blood, cortisol binds to CBG.
Free hormone portion enters target cells, binds to its receptor, and stimulates or inhibits gene transcription.
All cells are potential targets:
Permissive action: activates transcription of several enzymes that facilitate the action of other hormones (e.g. glucagon).
Stress hormone: cortisol levels increase during stress, enhances effect of norepinephrine on blood pressure, stimulates gluconeogenesis, inhibits glucose utilization → high plasma glucose concentration.
At high level, stimulates degradation of fat and protein → high plasma AA and FA concentration.
Cortisol also inhibits gene transcription in many tissues. Combined with degradation of fat and protein = growth inhibition effect, energy conserved for survival during high stress.
Anti-inflammatory effects: inhibits formation of protaglandins and cytokines, reduces lymphocyte migration.