Covalent Bonds & Molecules
1/22
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
23 Terms
What is a covalent bond?
A bond that results in the sharing of valence electrons between non-metal atoms.
What is a diatomic element?
When two atoms of the same element are joined by a covalent bond.
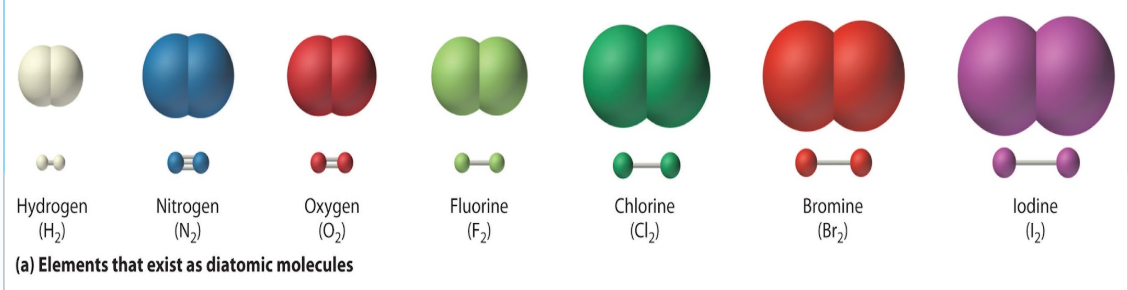
What is a molecular compound?
2 or more different atoms joined by covalent bonds.
Why can’t molecular compounds use the criss cross method?
The atoms can be combined in different ratios. This also means NO REDUCING.
How many atoms does “mono” indicate?
1
How many atoms does “di” indicate?
2
How many atoms does “tri” indicate?
3
How many atoms does “tetra” indicate?
4
How many atoms does “penta” indicate?
5
How many atoms does “hexa” indicate?
6
How many atoms does “hepta” indicate?
7
How many atoms does “octa” indicate?
8
How many atoms does “nona” indicate?
9
How many atoms does “deca” indicate?
10
What is the first step in naming a molecular compound?
Write the element that comes first. If that atom only has 1, you do not need to write “mono” infront of it. Otherwise, use the prefixes.
What is the second step in naming a molecular compound?
Write the second element and end with “ide”. Use prefixes.
What is the first step in writing the chemical formula for a molecular compound?
Write the chemical symbol in the order gieven in the name.
What is the second step in writing the chemical formula for a molecular compound?
Add the subscripts depending on the prefixes. NEVER SIMPLIFY.
What is the proper name for NH3?
Ammonia
What is the proper name for H2O?
Water
What is the proper name for O3?
Ozone
What is the proper name for CH4?
Methane
How to name a diatomic?
Name then usually the state (ex: gas)