Biology Final Exam
1/102
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
103 Terms
Titanium has an atomic number of 22. How many protons, neutrons, and electrons are in an isotope of titanium with mass number of 48?
p = 22, n = 26, e = 22
What type of bond is very prevalent in lipids and gives lipids their hydrophobic properties?
nonpolar covalent
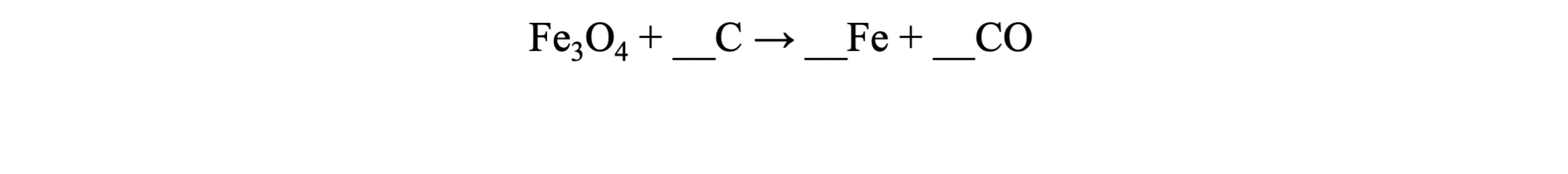
What numbers must be placed as coefficients in the blanks for the chemical reaction below in order to ensure that matter is conserved?
4, 3, 4
What would an electron holding extra energy be useful for?
A. powering a biochemical process that requires energy
b. storing energy for a time
c. stabilizing a lipid molecule
d. a and b
e. a and c
d. a and b
Which of the following is a product of photosynthesis that is a reactant in a campfire?
Oxygen
What are the four emergent properties of water that are important for life?
cohesion, moderation of temperature, expansion upon freezing, solvent properties
Water shows high cohesion and surface tension and can absorb large amounts of heat because of large numbers of which type of bonds between water molecules?
hydrogen bonds
If water was treated with molecules that reduce or remove surface tension, which of the following would occur?
A. Treated water droplets would form a thin film instead of beading on a waxed surface
b. Treated water would form smaller droplets when dripping from a sink
c. Water striders would sink
d. All of the above would occur
e. Only a and c would occur
d. all of the above
What is the concentration of hydroxide ions in a solution where pH = 10?
1 × 10–4 M
Which of the following acts as a pH buffer in blood?
A. carbonic acid
b. bicarbonate ion
c. carbonate ion
d. hydroxide ion
e. a and b
e. a and b
Which of the following explains how a pool of water can crack a boulder sitting in it during freezing weather?
expansion of the water when it freezes
What type of chemical bond joins a functional group to the carbon skeleton of a large molecule?
covalent bond
Which functional group is not found in biological organic molecules?
Cyanate
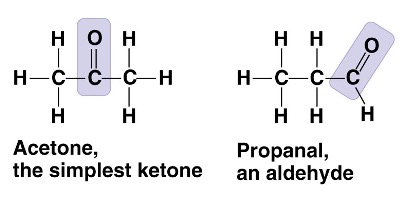
What type of isomer is propanal compared to acetone?
structural isomer
Which type of molecule may contain sulfhydryl groups?
Protein
What kind of bond would be present in a completely flat section of a biological molecule?
A. single bond
b. double bond
c. triple bond
d. b or c
e. a or c
d. b or c
If you swapped a methyl group for every phosphate group in a DNA molecule,
the molecule would fall apart
Which polysaccharide has the greatest number of branches?
Glycogen
A polysaccharide you are studying contains unbranched β glucose molecules and cannot be digested by humans. Which polysaccharide are you studying?
Cellulose
Lipids cannot be considered polymers because
they are not composed of monomer subunits
The chemical bonds present in a molecule contribute to the properties of the molecule. Carbon is an unusual atom in that it can form multiple bonds. Which statement is not true?
Saturated fats are those that have carbon-to-carbon double bonds and are associated with good health
All lipids
do not dissolve well in water
How does RNA differ from DNA?
DNA contains thymine, RNA contains uracil.
If you wanted to design a new industrial catalyst based on something biological, which molecule would you use?
Protein
If you substituted alanine for glycine in a protein, how much change to the protein’s structure would you expect?
little change, since these are both hydrophobic amino acids
Which structure is common to all three domains of life?
phospholipid bilayer cell membrane
Which of the following correctly lists the objects in order from largest to smallest?
human body, frog egg, mitochondrion, lipid
Which best describes a biological membrane?
two layers of phospholipids with proteins either spanning the layers or on the surface of the layers
A correct distinction between facilitated diffusion and active transport is that
facilitated diffusion depends on an existing energy gradient acting on the transported substance, while active transport makes such a gradient.
You are studying a pump protein in the lab. What should you order to make sure that the pump will operate?
ATP
Are most chemical reactions in living cells at equilibrium?
No
A reaction has a ∆G of –5.6 kcal/mol. Which of the following would most likely be true?
The reaction would proceed by itself but might be very slow
The oxidation of glucose to CO2 and H2O is highly exergonic: ∆G = –636 kcal/mole. This is spontaneous, but why is it very slow?
Few glucose and oxygen molecules have the activation energy at room temperature
Vioxx and other prescription nonsteroidal anti-inflammatory drugs (NSAIDs) are potent inhibitors of the cyclooxygenase-2 (COX-2) enzyme. High substrate concentrations reduce the efficacy of inhibition by these drugs. These drugs are
competitive inhibitors
Some enzymes can couple the hydrolysis of ATP to ion transport by having
changes during ATP hydrolysis alter the enzyme’s shape, forcing ionic transport to occur
How are the effects of feedback inhibition and allosteric factors similar to how enzymes often couple reactions?
All often involve inducing structural changes in the enzyme, influencing its activities.
A friend is designing an industrial process around an endergonic biological reaction. You recommend finding a way to provide
ATP.
What can cause a reaction to occur spontaneously?
–∆G
Cellular respiration can best be described as
taking electrons from food and giving them to oxygen to make water, using the energy released to drive ATP formation.
Drugs known as uncouplers facilitate diffusion of protons across the membrane. With an uncoupler, what will happen to ATP synthesis and oxygen consumption if the rates of glycolysis and the citric acid cycle stay the same?
ATP synthesis will decrease oxygen consumption will stay roughly the same.
The hydrogens taken from glucose or a breakdown product of glucose are added to oxygen, releasing energy to
actively transport H+ into the intermembrane space.
ATP synthase at the inner mitochondrial membrane makes ATP and water from ADP and phosphate by coupling this to which other process?
allowing H+ to move down its electrochemical gradient
Which of the following is not an immediate net product of the typical mitochondrial electron transport chain?
ATP
You are handed a biochemical extract from cells that were performing cellular respiration. You detect cytochromes in one fraction, so it was probably used for the study of
electron transport.
How are photosynthesis and cellular respiration connected?
The first produces glucose, full of energy, and the second extracts that energy
Of these events from the light reactions, which occurs first?
Light-induced reduction of the primary electron acceptor in the reaction center of PS II
When donating its activated electron, the chlorophyll in photosystem II is a very powerful oxidizing agent. This is best shown by its ability to
force the oxidation of oxygen in water to oxygen gas
One reason for carrying out the production of oxygen gas in the space surrounded by the thylakoid membranes, and not in the stroma of the chloroplasts, is
that the hydrogen ions released can contribute to the H+ electrochemical gradient being generated
The enzyme rubisco catalyzes the fixation of carbon. Considering all the carbons involved, is the production of 3-PGA a net oxidation, reduction, or neither? Why?
Neither. There is no change in the total C–O and C–H bonds between the products and reactants
If a cell contained a radioactive version of rubisco, where would the radioactivity be located?
Stroma
Which best links photosynthesis and cellular respiration?
Chemiosmosis
Which comes immediately after S phase in the cell cycle?
G2
You are observing a line of rat cells and see that they repeatedly make mistakes in the cell cycle by going through the G2 checkpoint too early. This could be due to a
problem with expression of a cyclin
Binary fission is more like animal cell division than plant cell division because
bacteria and animals both pinch in to separate the cytoplasm into two pieces
Why is it more practical to prepare karyotypes by viewing somatic diploid cells rather than haploid gametes?
Both sets of chromosomes, which are present in somatic diploid cells, need to be examined.
Crossing over begins to occur during
prophase I
Why does sexual reproduction (via meiosis) have an advantage over asexual reproduction (via mitosis)?
Meiosis increases genetic variation among offspring
A pea plant is heterozygous at the independent loci for flower color (Pp) and seed color (Yy). What types of gametes can it produce?
four gamete types: pY, py, PY, and Py
A cross between homozygous purple-flowered and homozygous white-flowered pea plants results in offspring with purple flowers. This demonstrates
dominance
The following offspring were observed from many crossings of the same pea plants. What genotypes were the parents? 465 purple axial flowers 152 purple terminal flowers 140 white axial flowers 53 white terminal flowers
PpAa × PpAa
In peas, the allele for tall stems (T) is dominant to that for dwarf stems (t), and the allele for axial flowers (A) is dominant to that for terminal flowers (a). A plant of unknown genotype with tall stems and axial flowers is crossed with a plant with dwarf stems and terminal flowers. Among the offspring are 38 plants with tall stems and axial flowers, and 36 plants with tall stems and terminal flowers. What is the previously unknown genotype?
TTAa
Morgan and his colleagues worked out a set of symbols to represent fly genotypes. Which of the following is representative?
Xw+ or Xw
In some species of Drosophila, there are genes on the Y chromosome that are not on the X chromosome. Imagine that a new allele arises on the Y chromosome and reduces the size by half of individuals with the new allele. Which of the following statements is accurate with regard to this situation?
This allele is passed to all male but no female offspring of a male with the allele
In cats, an X-linked gene affects coat color. The O allele produces an enzyme that converts eumelanin, a black or brown pigment, into phaeomelanin, an orange pigment. The o allele is recessive to O and produces a defective enzyme, one that does not convert eumelanin into phaeomelanin. Which of the following statements is accurate?
The phenotype of o-Y males is black/brown because the nonfunctional allele o does not convert eumelanin into phaeomelanin
In Drosophila, white eyes are due to an X-linked recessive allele (Xw). Which of the following crosses could not result in a white-eyed Drosophila male?
homozygous red-eyed females with white-eyed males
How do the leading and the lagging strands differ?
The leading strand is synthesized in the same direction as the movement of the replication fork, whereas the lagging strand is synthesized in the opposite direction
What enzyme compensates for replication-associated shortening of linear chromosomes?
Telomerase
Suppose a double-stranded DNA molecule was shown to have 22% guanine bases. What would be the expected percentage of adenine bases in that molecule?
28%
In Meselson and Stahl’s experiment proving semiconservative DNA replication, they started with bacteria grown in a heavy isotope of nitrogen and then switched them to a light isotope. They then observed the DNA density after one and two rounds of replication. What was the result after one round of replication?
all hybrid DNA
In Meselson and Stahl’s experiment proving semiconservative DNA replication, they started with bacteria grown in a heavy isotope of nitrogen and then switched them to a light isotope. They then observed the DNA density after one and two rounds of replication. What was the result after two rounds of replication?
equal amounts of light and hybrid DNA
In Meselson and Stahl’s experiment proving semiconservative DNA replication, they started with bacteria grown in a heavy isotope of nitrogen and then switched them to a light isotope. They then observed the DNA density after one and two rounds of replication. If they had measured after a third round of replication, what would they have observed?
three times as much light as hybrid DNA
Imagine a bacterial replication fork. Synthesis of which new strand(s) would be affected by mutations in the enzyme DNA polymerase III?
both leading and lagging strands
Imagine a bacterial replication fork. Synthesis of which new strand(s) would be affected by mutations in the enzyme primase?
both leading and lagging strands
Imagine a bacterial replication fork. Synthesis of which new strand(s) would be affected by mutations in the enzyme DNA ligase?
Does the distribution of bases in sea urchin DNA and salmon DNA follow Chargaff’s rules?
Yes, because the %A approximately equals the %T and the %G approximately equals the %C in both species.
The template strand of a given gene includes the sequence 3′-GCCACGTATCA-5′ What is the sequence of the nontemplate strand?
5′-CGGTGCATAGT-3′
Which of the following is the best example of gene expression?
Mouse fur color results from pigment formed by gene-encoded enzymes
Which of the following components does not form part of the transcription initiation complex at a eukaryotic promoter?
transfer RNA
Which of the following is a modification made to eukaryotic mRNA before it is exported to the cytosol?
A. The 5′ end is capped.
B. A poly-A tail is added to the 3′ end
c. introns are removed
d. Exons are joined together
e. all of the above
e. all of the above
Aminoacyl-tRNA synthetase is an enzyme whose function is to _____
link a tRNA to its corresponding amino acid
The codon for the amino acid Trp in 5’ to 3’ direction is UGG. What is the 5′ → 3′ sequence on the anticodon of the (Trp) tRNA?
CCA
Which of the following is not a normal feature of prokaryotic mRNA?
poly-A tail
What does the operon model attempt to explain?
the coordinated control of gene expression in bacteria
When tryptophan (an amino acid) is present in the external medium, the E. coli bacterium brings in tryptophan molecules and does not need to make them. Which of the following statements about the trp operon is true when there is no tryptophan in the medium?
The repressor is inactive, and RNA polymerase can synthesis mRNA
Each of a group of E. coli bacterial cells has a different disabling mutation in its lac operon. Which of the following will make it impossible for the cell to metabolize lactose?
mutation in lac (-galactosidase gene)
A specific gene is known to code for three different but related proteins. This could be due to which of the following?
alternative RNA splicing
Even though both lens cells and liver cells have numerous transcription factors that are present in both cells, the lens cell makes the crystallin protein (not albumin), whereas the liver cell makes albumin (not crystallin). Which of the following explains this cell specificity?
Different specific transcription factors made in each cell determine which genes are expressed
Imagine an E. coli cell with a mutation that renders its lac repressor protein completely inactive. Which of the following would be true of that cell?
Always synthesize β-galactosidase
Imagine an E. coli cell in which the trp operator has a mutation that renders it unable to bind the trp repressor. Under what circumstances would its trp operon be “on”?
It would always be on
Histone acetylases are enzymes that add acetyl groups to histone proteins. In what way do histone acetylases affect transcription?
Activate transcription by opening chromatin
Which gives the most complete and correct description of a signal transduction pathway?
sequence of changes in a series of molecules resulting in a response
A steroid hormone is bound by an intracellular receptor. The resulting complex is most likely to do what?
act as a transcription factor in the nucleus
Which correctly describes what happens during signal transduction inside a cell?
Changes in protein activities and in other items in the cell are induced by an activated receptor
Which can be second messengers?
A. Ca2+
b. cAMP
c. DAG
d. a and b
e. a, b, and c
e. a, b, and c
If you wanted to block signaling involving a steroid molecule, which should you destroy?
nuclear pores
Which would cause the most problems for a cell-signaling pathway involving a steroid molecule?
lack of Ca2+
Which would cause the most problems for a cell-signaling pathway involving a G protein-coupled receptor?
not enough GTP in the cell
Hormone pathways involved in maintaining homeostasis (such as the secretin pathway in the digestive tract) are often characterized by which of the following?
negative feedback
Which of the following is not a product of the anterior pituitary gland?
thyrotropin-releasing hormone
Which of the following is not an effect of catecholamines?
suppression of the immune system