6. viral genetics/evolution + antiviral drugs
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
what are the mechanisms of viral evolution? (3)
genetic drift (gradual)
genetic shift (abrupt)
recombination (abrupt)
genetic drift
point mutations
error occurs during copying of DNA or RNA, leading to changes in the nucleic acid sequence
gradual
antigenic drift
amino acid sequence changes due to genetic drift (mutation); will result in a change in the protein or viral antigen
nucleotide insertion/deletion
may be single nucleotide or larger stretches
causes frameshift → altered protein sequences, incorrect amino acids
dysfunctional, nonfunctional, truncated protein = detrimental to virus
why do RNA viral polymerases have high mutation rates?
more errors due to minimal to no proofreading and editing enzymes
genetic shift
exchange of viral gene segments between two virus strains to generate a new virus strain
genetic reassortments
abrupt
antigenic shift
when genetic shift results in different viral antigens (what immune system will recognize)
what are the two important requirements for genetic shift?
viral genome has to be segmented
two related viruses must infect the same host and cell
ex. pigs have receptors for both avian and human influenza viruses → act as mixing vessel for influenza viruses
recombination
an exchange of part of the viral genome with that of another virus
splicing genomes
abrupt
very common with DNA viruses (can also occur with RNA viruses)
not the same as reassortment → does not require segmented genomes
recombination can occur between _____
same type of viruses (ex. two herpesviruses; HSV-1 & HSV-2)
two different, but related viruses (same family)
what are consequences of viral evolution?
potential change in fitness
deleterious mutations = defective replication or less fit virus compared to parent; no survival
increased virulence
changes in tropism and transmission efficiency
immune escape
drug resistance
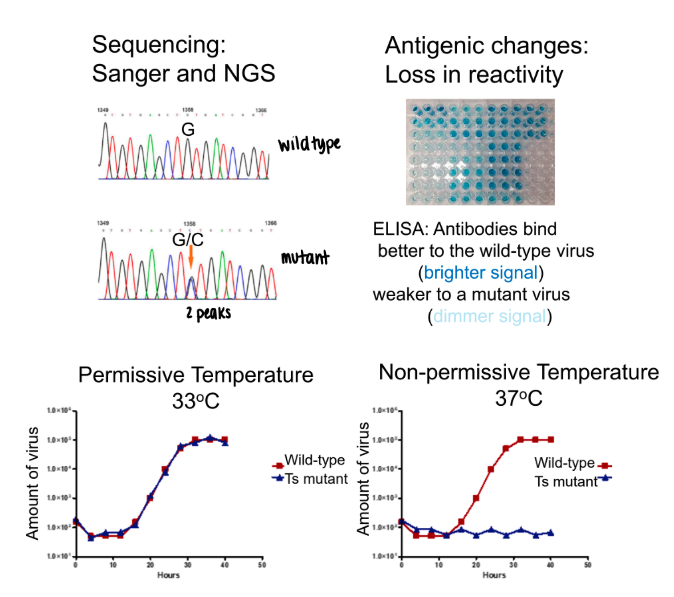
what are examples of methods to detect/identify mutations?
sequencing: sanger and NGS
antigenic changes: loss in reactivity
ELISA: antibodies bind better to wild-type virus (brighter signal); weaker to mutant virus
phenotypic changes: temperature sensitive mutations
qualities of an ideal antiviral drug
broad-spectrum antiviral → one drug targets multiple viruses
minimal toxicity
multiple animal species
side effects should not cause greater illness than the virus
what are the targets for host-targeting antiviral drugs?
host proteins that are required for the virus life cycle
advantages of host-targeting antiviral drugs
broad-spectrum potential
reduced risk of drug resistance
limitations/disadvantages of host-targeting antiviral drugs
species-specific potential
greater risks of cellular toxicity → concern targeting host protein; essential for the cell
host ribosome — essential for host and virus = not a good target
broad-spectrum
cellular toxicity
host receptor — not completely essential for host
not broad-spectrum
tolerable
what are the targets for direct-acting antiviral drugs?
any viral protein in any step of the virus life cycle
advantages of direct-acting antiviral drugs
highly specific/reduced toxicity (RT and RdRp proteins — unique to viruses)
fast-acting → directly inhibiting a viral process
limitations/disadvantages of direct-acting antiviral drugs
narrow spectrum
resistant mutant viruses → can take only 1 mutation to become resistant
some viral RNA polymerases have a higher error rate → rapid antiviral resistance
however, mutations come at a cost to the virus (gain resistance vs. fitness loss)
what is neuraminidase’s (NA) function (influenza virus)?
during budding, new virions can bind to host cell surface receptors → NA cleaves receptors to release virion → continued infection and replication
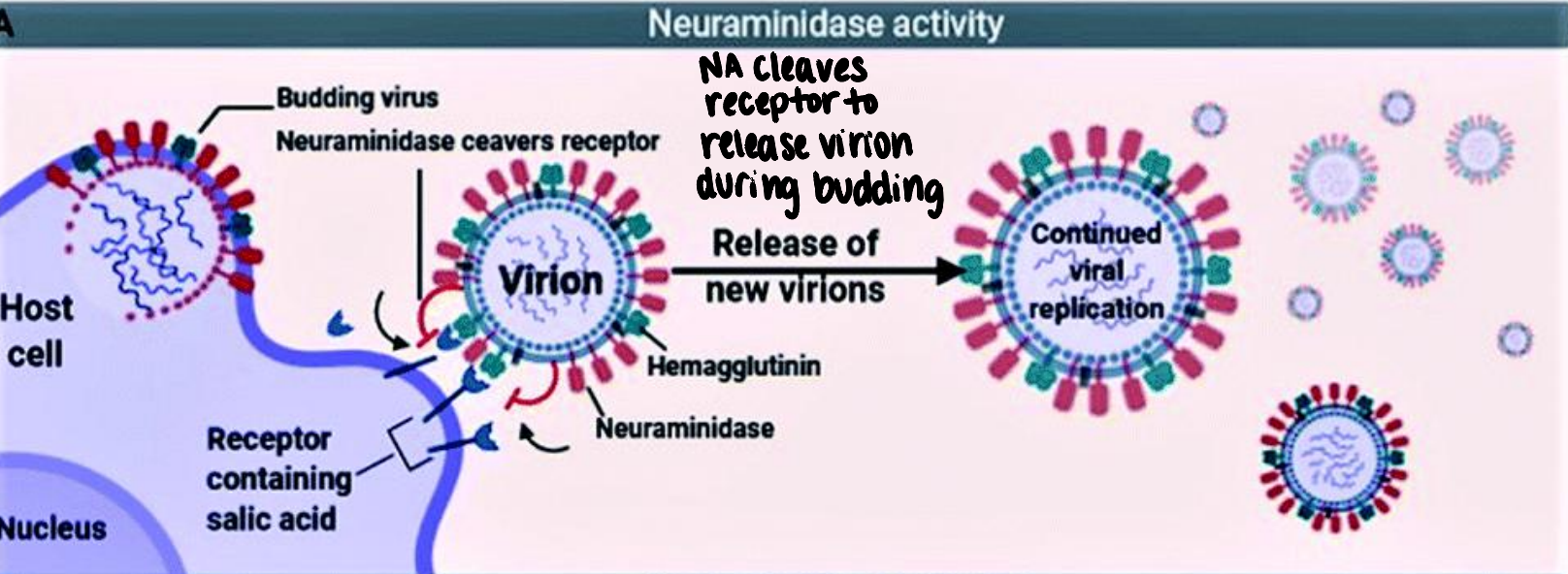
how do neuraminidase inhibitors work?
bind NA → NA cannot cleave receptor → budding virion stuck to host cell
how do nucleotide analogs prevent replication?
lack a 3’ OH group → when incorporated into string of nucleotides, polymerases cannot add another nucleotide on → termination of elongation
obstacles to antiviral use in veterinary medicine
toxicity/bioavailability
side effects
species differences
resistance
high mutation rates, especially in RNA viruses
highly specific
difficult to create “broad spectrum” drugs
$ (treatment very costly)