chemical energetics
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
18 Terms
in a chemical reation broken bonds….
in a chemical reaction, bonds of reactant molecules are bring broken, while new bonds are being created with the broken reactant bonds
endothermic, bond breaking
more energy is absorbed in breaking bonds, than forming bonds
bond enthalpy
average amount of heat absorbed to break one mole of that chemical bond in the gaseous state
enthalpy (H)
a measure total heat energy in a thermodynamic system
enthalpy change (△H)
a change in total heat energy in a thermodynamic system in an object ( positive or negative
enthalpy change instrument
enthalpy change can be measured using a calorimeter
enthalpy change formula
△H = total energy of products - total energy of reactants
exothermic, bbf
more energy is released in forming bonds, than breaking bonds
bond breaking formula
△H = total energy absorbed for bond breaking - total energy released from bond breaking
if neg (exo)
if positive (endo)
requirement for bond breaking
higher energy absorbed is needed to break stronger bonds
bond enthalpy formula
△H = total energy absorbed for bond breaking - total energy released from bond forming
activation energy explained 2
for the reactants to collide successfully the particles must react with the correct orientation
2 types of enthalpy changes
exothermic reaction ( - )
endothermic reaction ( + )
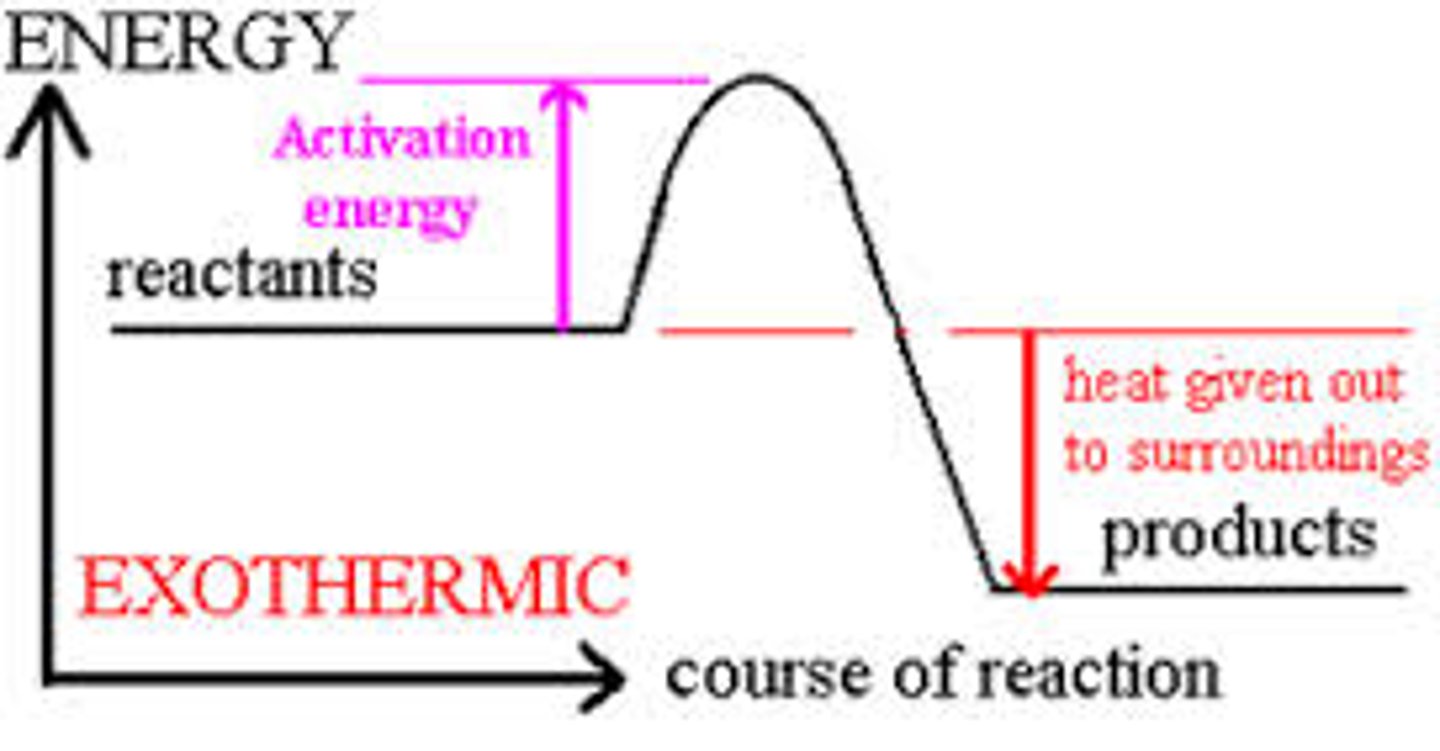
exothermic reaction
loses energy in the reaction ( - )
object cools down
surroundings gets warmer
object released internal energy to surroundings
endothermic reaction
gains energy in the reaction ( + )
objects gets warmer
surroundings gets cooler
object absorbs the heat from the surroundings
activation energy
the minimum energy that is needed by the reactant particles for a chemical reaction to occur
activation energy explained
when reactant particles collide with each other to react, for the reaction to occur a certain amount of energy is required ( activation energy )
energy profile diagram
diagram that shows the energy used over time with enthalpy change (△H) and activation energy