molecular orbital theory
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
18 Terms
what are molecular orbitals
they describe the way in which multiple electrons can move around more than one nucleus
how are the molecular orbitals generated
linear combination of atomic orbitals (LCAO) method
atomic orbitals interact with each other to form MOs in a way that can be thought of as constructive and destructive interference
how many MOs are generated
number of AOs at start = number of MOs at end
what are the combinations of atomic orbitals and what MOs do they form
x + y linear combination forms the bonding MO (σ/π) at lower energy than the AOs
x - y linear combination forms the antibonding MO (σ*/π*) at higher energy than the AOs
px orbital

py orbital
pz orbital
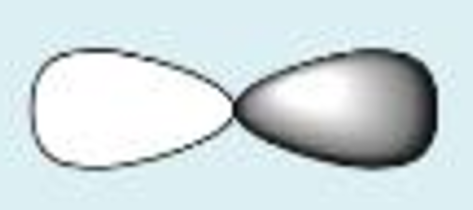
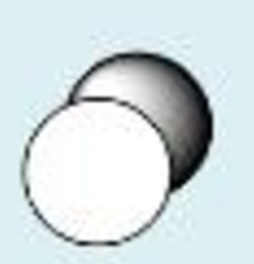
s + s constructive
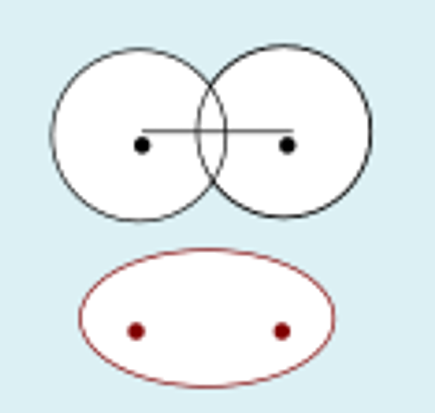
s + s destructive
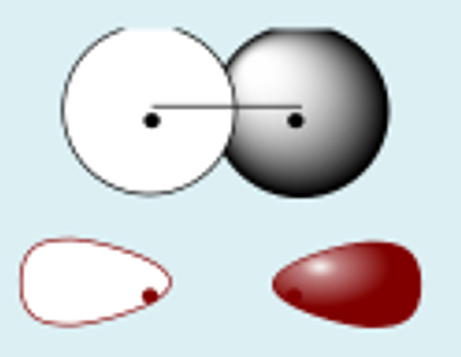
s + pz constructive
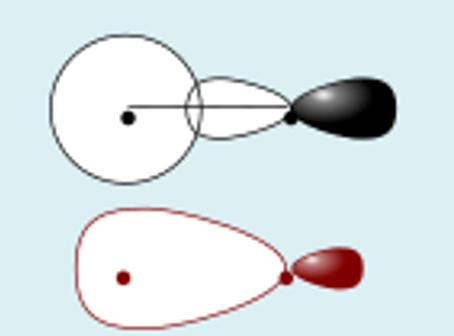
s + pz destructive
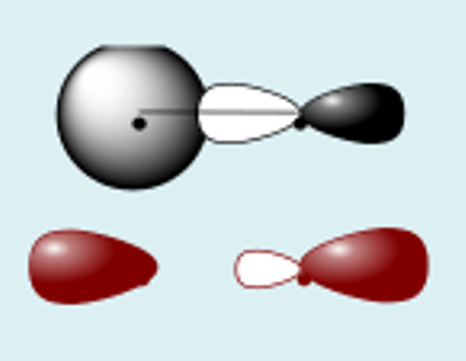
px + px constructive
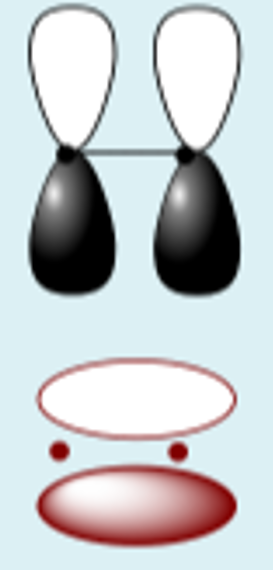
px + px destructive
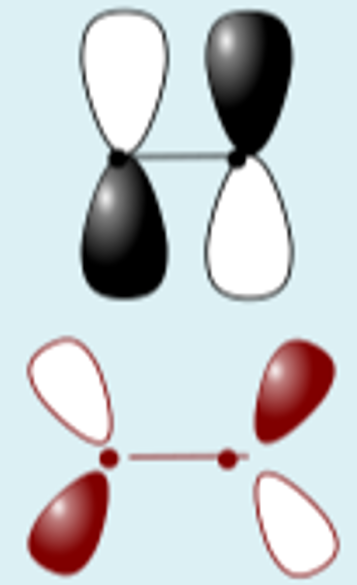
py + py constructive
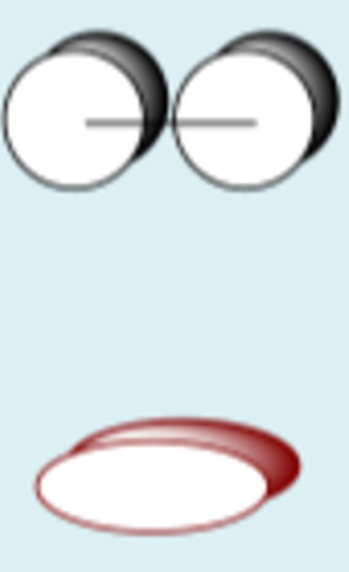
py + py destructive
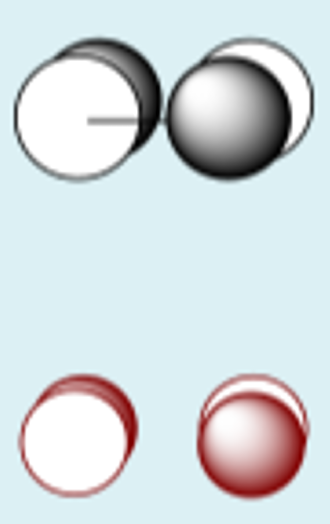
pz + pz constructive
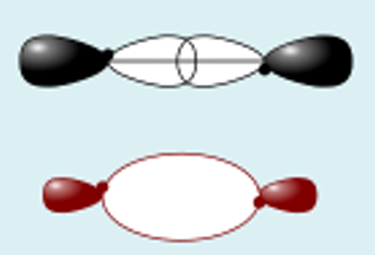
pz + pz destructive
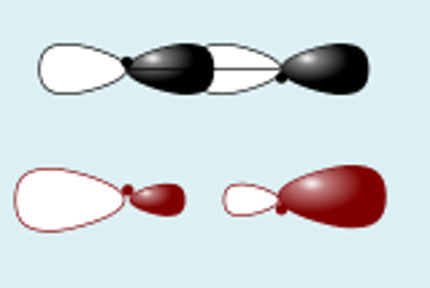
how to calculate bond order
(bonding electrons - antibonding electrons)/2