Ch 10 learning objectives Lipid bilayer & Membrane proteins
1/39
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
40 Terms
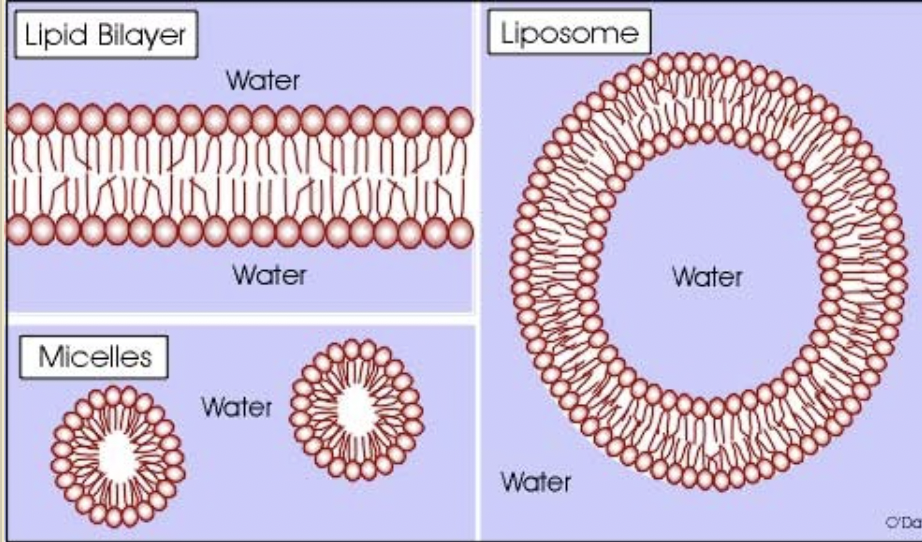
Amphipathic lipids
contain a nonpolar domain (hydrophobic tails)
has polar domain (hydrophilic heads)
allows is to associate w/ “like” environments , forming bilayers, micelles, liposomes
Non polar Lipids
any portions of a lipid that has non polar bonds
bonds w/ small EN differences , ex: CH
diff: 0 to 0.4 EN
Polar lipids
any portions of a lipids that have polar bonds
bonds w/ larger EN differences , ex: OH
diff: 0.41 to 1 EN
what are the main phospholipids in most animal cell membranes
glycerophospholipids yelin= external
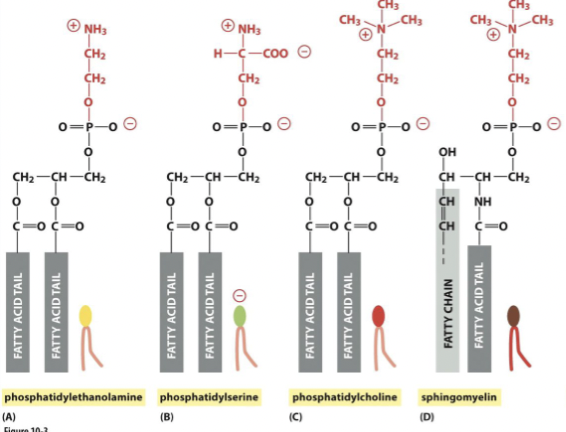
what are the 4 most common glycerophospholipids in mammalian membranesyin
phosphatidylserine (PE)= internal
phosphatidylserine (PS) = internal, neg. charged
phosphatidylcholine (PC) = external
sphingomyelin = extrenal
saturated vs unsaturated lipids
saturated = single bonds
unsaturated = has at least one double bond
Factors affecting membrane fluidity
Composition
Cholesterol: makes lipids stiff; less fluid
saturated fatty acids: less fluid
unsaturated fatty acids: more fluid
Length
short chains are more fluid
temperature
more fluid at high temperatures
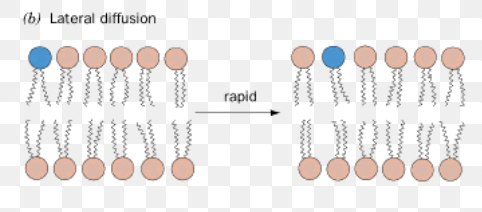
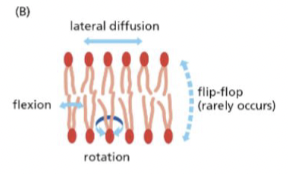
Lateral diffusion
movement within the plane of the leaflet
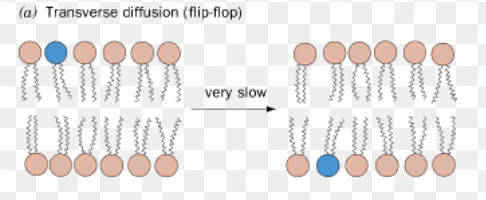
Flip Flop ( transverse diffusion)
lipids switch to different leaflet sides
needs the help of flippases, scamblases, & phospholipid translocases
cholesterol easily flip flops
Flexion
tails can flex and move
rotation
lipids can rotate
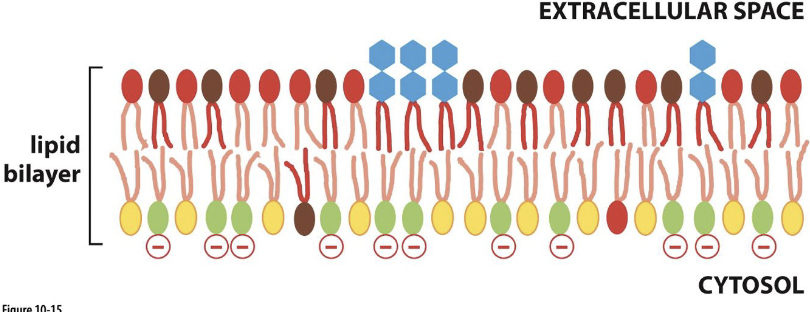
Why does asymmetry matter?
membrane change:
presence of phosphatidylserine (PS) on extracellular side signals cell death
cell signaling:
PS and phosphatidylinositol (PI) (also found on cytosolic side) bind intracellular signaling proteins
membrane lipid aggregation
when lipids cluster together in patches / aggregates b/c they attract each other more than others
These lipid aggregates can affect membrane’s flexibility, permeability, and ability to interact with other molecules.
This process is essential for cell signaling, membrane trafficking, and maintaining cell structure.
Lipid rafts
form in areas of greater fluidity (more saturated lipids)
attracts transmembrane proteins w/ longer hydrophobic transmembrane domains
important for cell signaling
composed of
cholesterol
saturated hydrocarbons
glycolipids
carbohyrate groups are always on the non-cytosolic side
how does lipid aggregation & rafts affect cell signaling
serve as platforms or "rafts" where signaling molecules (ex: receptors & enzymes) concentrate ; facilitate the efficient assembly of signaling complexes, allowing for more effective signal transduction
By clustering specific lipids and associated proteins together, they create microdomains where signaling molecules can interact more readily
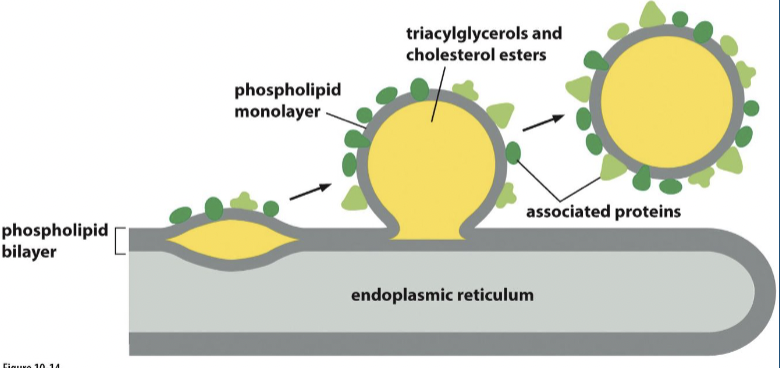
Lipid droplet
storage for excess lipid from where they can be retrieved as building blocks for membrane synthesis or as a food source fueling metabolic energy generation
surrounded by a phospholipid monolayer
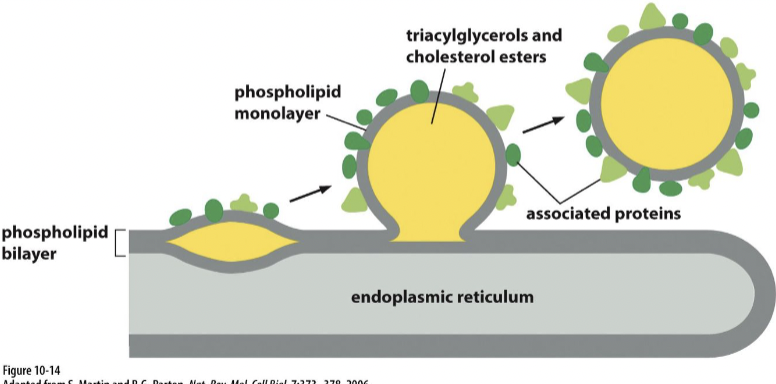
formation of lipid droplet
through a process involving the accumulation and packaging of neutral lipids, such as triglycerides and cholesterol esters.
starts with :
Lipid Synthesis: Neutral lipids are synthesized within the ER membrane, primarily through enzymatic reactions involving fatty acids and glycerol.
Lipid Accumulation: As neutral lipids accumulate within the ER membrane, they start to form small aggregates in the interleaflet space of the membrane.
Budding and Sequestration: These lipid aggregates gradually coalesce and bud off from the ER membrane, forming small droplets within the cytoplasm. This process involves specific proteins, such as lipid droplet-associated proteins, which aid in the budding and stabilization of the lipid droplets.
Maturation: Once formed, lipid droplets can grow in size by continued incorporation of neutral lipids synthesized within the ER or by lipid uptake from the surrounding environment
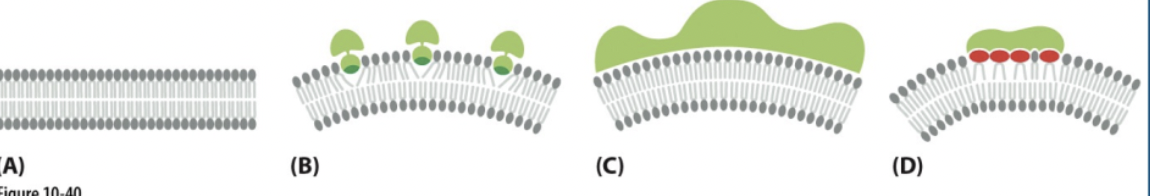
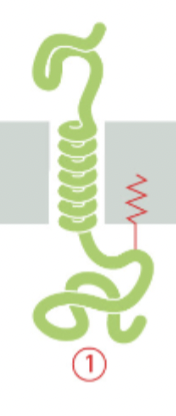
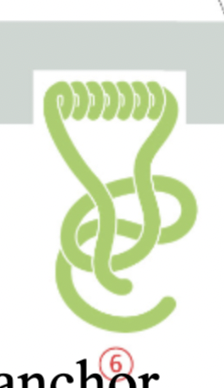
single-pass α-helix with lipid anchor
The α-helical region provides the hydrophobicity necessary for insertion into the lipid bilayer
while the lipid anchor enhances membrane association and stability.
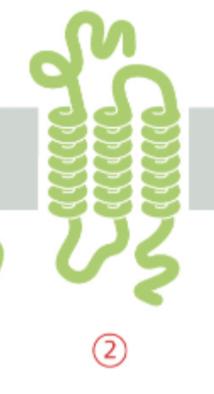
multipass protein
spans the lipid bilayer multiple times, in a "snake-like" manner
facilitating the transport of molecules across membranes, signal transduction, cell adhesion
has multiple helixes
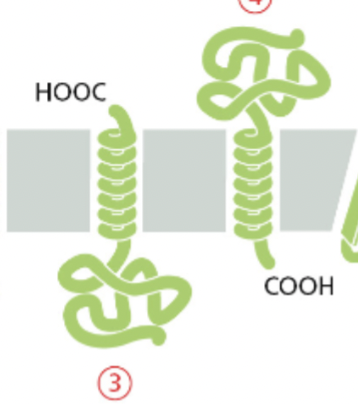
single pass transmembrane proteins
they have COOH attached
it has a helix
beta barrel (rolled up beta sheet)
this is a bundle of multiple beta strands connect by hydrogen bonds & wraps around to form a close loop

alpha helix inserted in only one leaflet of the lipid bilayer
this one is hydrophobic which is why its embedded in the hydrophobic region
lipid anchored protein
attached to the lipid bilayer through covalent attachment to lipid molecules
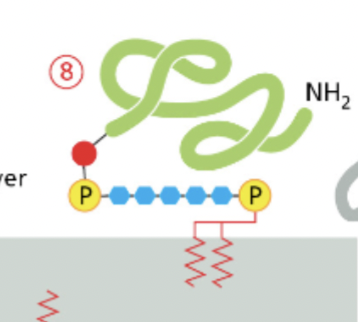
GPI anchored protein
are inserted into the outer leaflet of the lipid bilayer during their biosynthesis in the endoplasmic reticulum (ER)
first modified with GPI then inserted
attached to the outer leaflet of the plasma membrane through a GPI moiety.
This anchor consists of a complex glycolipid structure that is attached to the C-terminus of the protein.
GPI-anchored proteins play roles in cell signaling, cell adhesion, and immune response.
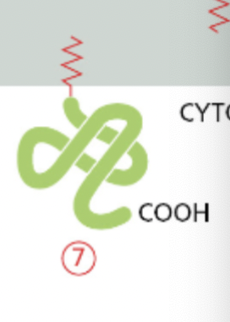
Peripheral membrane proteins
associate with the membrane through non-covalent interactions with other membrane-associated proteins or with lipid molecules
on the cytoplasmic side or the extracellular side.

integral vs lipid anchored vs peripheral proteins
integral = #1,2,3,4,5,6 ← in the bilayer
canNOT be extracted from the membrane by high salt concentrations or changes in the pH
lipid anchored = #7, 8 ← attached to the outside covalently
the covalent attachment helps localize water soluble protein to a membrane after its synthesis in the cytosol
peripheral = # 9 , 10 ← attached to the outside non- covalently
CAN be extracted from the membrane by high salt concentrations or changes in the pH
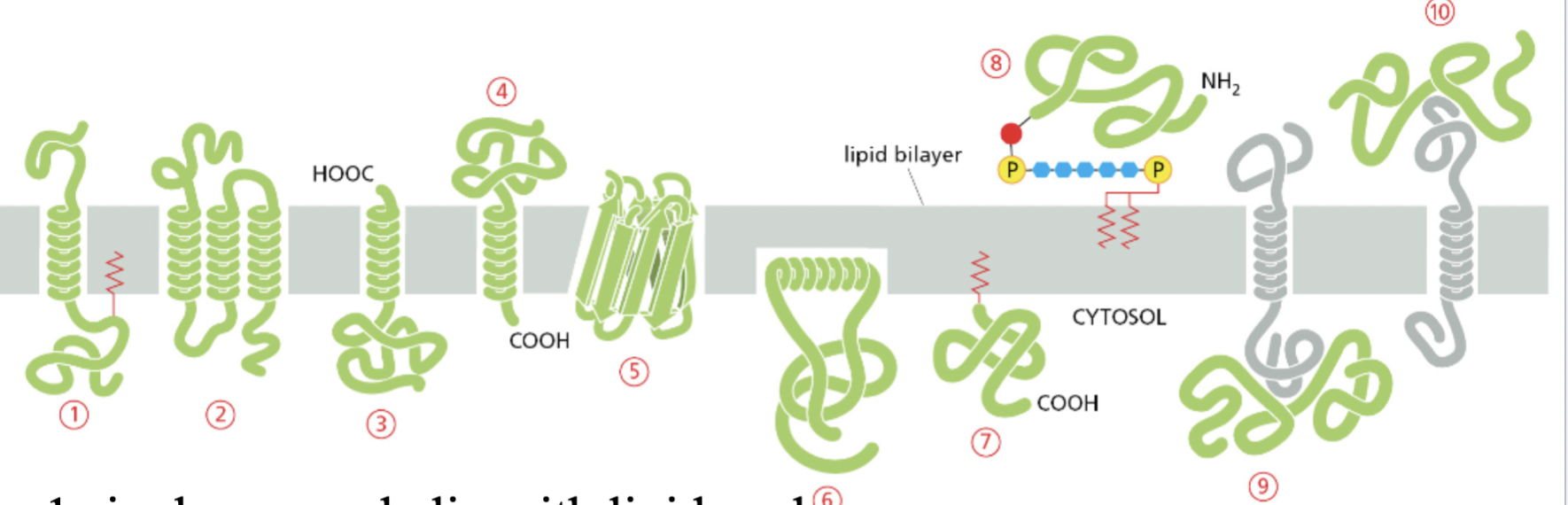
1. single pas a-helix w/ lipid anchor
multipass protein
Beta barrel
amphipathic
interact w/ both sides of the plasma membrane
a typical alpha helical transmembrane domain is composed of 20 -30 amino acids
beta sheet transmembrane domain is about 10 amino acids in length
proteins that are in membrane are ….
nonpolar amino acids
proteins that interact with cytosol or extracellular fluid are …
polar amino acids
Do normal rules for amino acid composition of spontaneously folding alpha helices apply?
No!
transmembrane proteins generally inserted by a translocator protein, which stabilize unfavorable helix components
Chaperones can also stabilize proteins & prevent premature folding
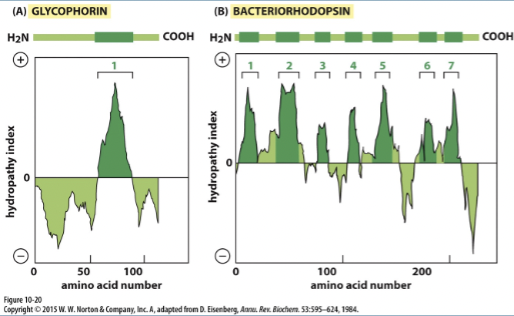
Hydropathy plots
hydropathy index
the hydrophobicity of an amino acid segment (positive hydropathy index = hydrophobic amino acid)
hydropathy plots: demonstrate presence of membrane spanning alpha helices
CANNOT identify membrane spanning beta sheets
high positive values or peaks indicate hydrophobic segments that are likely to form transmembrane helices.
reegions with negative values on the hydropathy plot correspond to hydrophilic segments that are typically located in the aqueous environment inside or outside the cell.
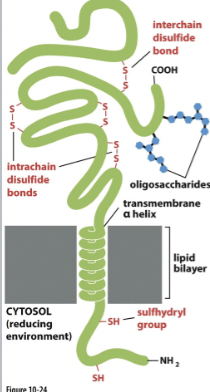
Reducing conditions
external cell environment: Non- reducing
allows formation of disulfide bonds
internal cell environment: Reducing
prevents formation of disulfide bonds
carbohydrate groups are found in the extracellular side of the plasma membrane
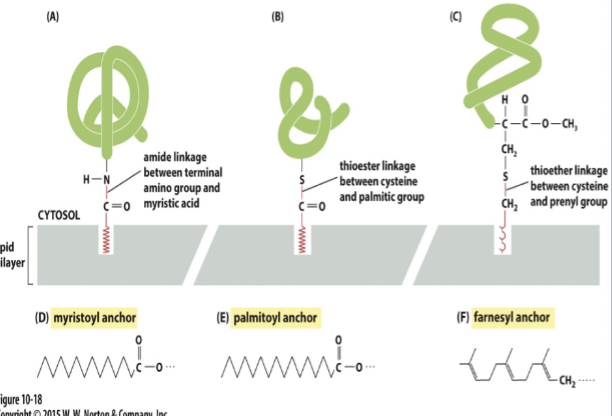
Lipid linked transmembrane proteins
attached to cytosolic face by:
fatty acid chains
prenyl groups (ex: farnesyl)
can be attached to the exoplasmic face by glycophosphatidylinosital (GPI) anchors
Detergents
amphipathic molecules used to solubilize integral membrane proteins
ionic detergents (ex: SDS)
Non-ionic detergents (ex: triton X-100 & Beta- octylgucoside)
break apart and dissolve lipids and membrane proteins by interacting w/ hydrophobic/ hydrophilic domains
“Cone shaped”
at low concentrations they exist as monomers
at high conc. they form micelles in water (Critical micelle concentration)
Critical micelle concentration (CMC)
when detergent molecules in a solution begin to aggregate and form micelles.
Micelles are spherical assemblies of detergent molecules arranged with their hydrophobic tails pointing inward and their hydrophilic heads facing outward
Chimeric cell studies
involve fusing two different types of cells, each labeled with different fluorescent markers. By observing the movement of these markers within the fused cell, researchers can infer the lateral diffusion of proteins or lipids in membranes. If proteins or lipids are free to move laterally within the membrane, then both markers should mix evenly throughout the fused cell over time. Conversely, if movement is restricted, distinct regions of the cell with different marker distributions will persist.
Chimeric cell studies compare the mixing of different markers between fused cells to assess lateral diffusion
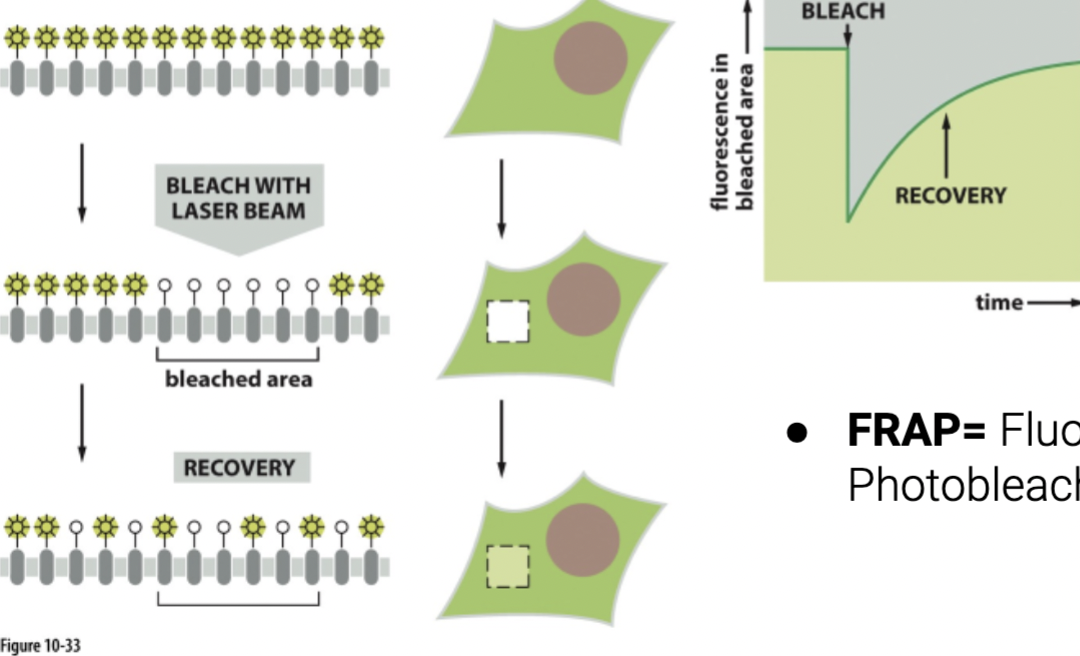
FRAP shows lateral diffusion by …
involve bleaching a small region of fluorescently labeled proteins or lipids within a cell membrane and then observing how quickly the fluorescence returns to the bleached area. If lateral diffusion is occurring, unbleached molecules from surrounding areas will move into the bleached region, resulting in a recovery of fluorescence over time. The rate of fluorescence recovery provides insights into the speed and extent of lateral diffusion in the membrane.
directly observe the recovery of fluorescence in a bleached area to measure the speed of lateral movement.
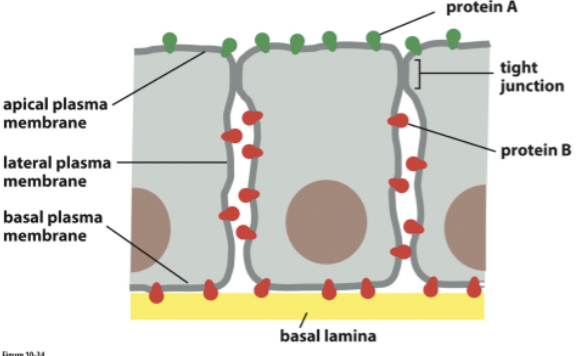
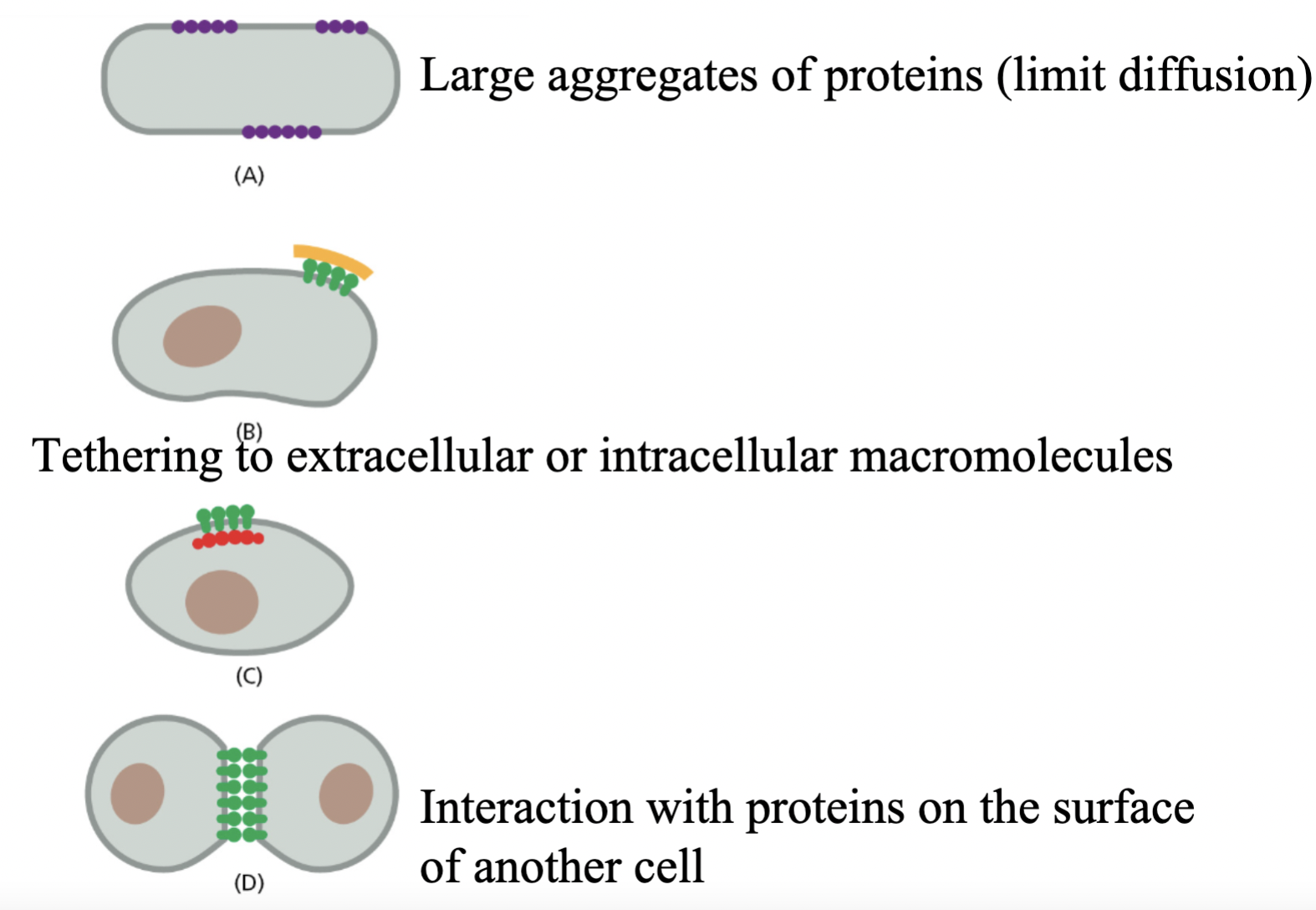
Ways diffusion is restricted:
aggregation of proteins
binding of molecules to extracellular matrix
binding to intracellular molecules (ex: cyto skeleton)
binding to molecules on other cells (ex: cell junctions)
tight junctions = connect cells and prevent molecules from moving between cells
membrane curvature
membrane protein & lipid composition can influence curvature
important for intracellular organelles that need to change shape to create transport vesicles
factors that cause curvature
insertion in one leaflet causes bending
curved structure causes bending
large polar lipid heads ( phosphatidylinositol)