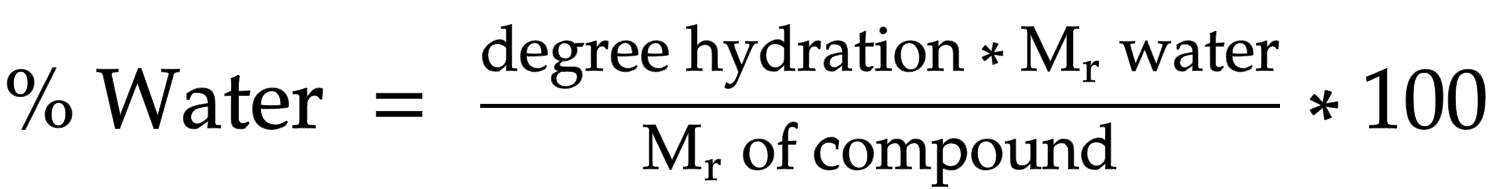1.7 Quantitative Chemistry 📝
1/28
Earn XP
Description and Tags
GCSE CCEA Specification GCSE Chemistry Double Award Science, Triple Award Science Unit 1: Structures, Trends, Chemical Reactions, Quantitative Chemistry and Analysis
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
29 Terms
Relative atomic mass (Ar)
mass of the atom compared to carbon-12 isotope (mass of exactly 12), and is weighted mean of mass numbers
Relative formula mass (Mr)
total relative atomic masses of atoms in the formula (ignore coefficient)
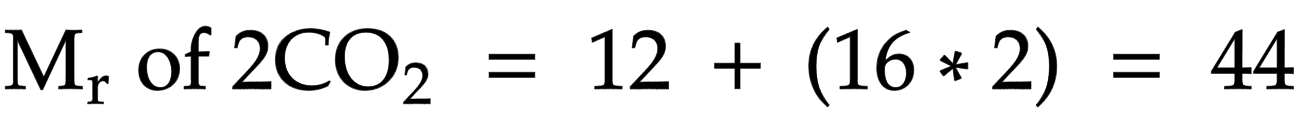
Percentage of element in compound
mass of element/ relative formula mass
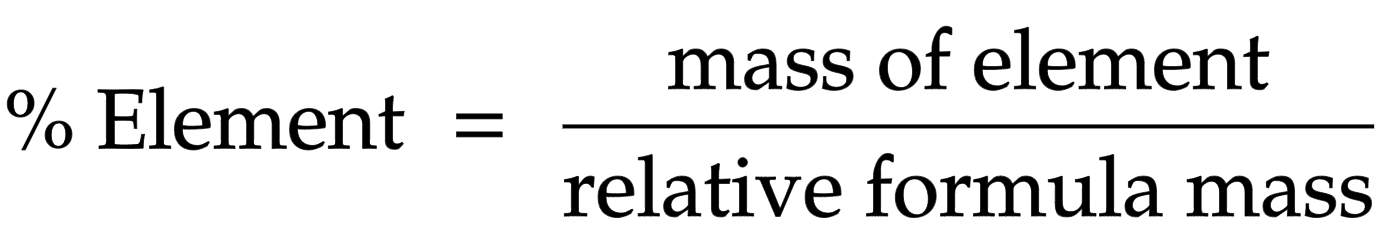
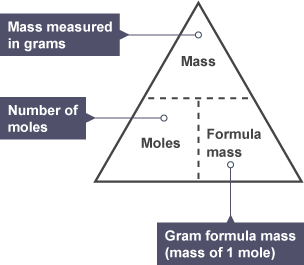
Mole (mol)
unit for measuring amount of a substance, 6.02×1023 particles
Mass of 1 mol in g
equivalent to relative formula mass e.g 12g carbon = 1 mol
Mole equation
moles = mass (g)/ Mr
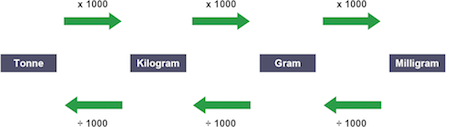
1 tonne
1000 kg

1 kg
1000 g
Molar ratio
in balanced equations this is the coefficient (big number)
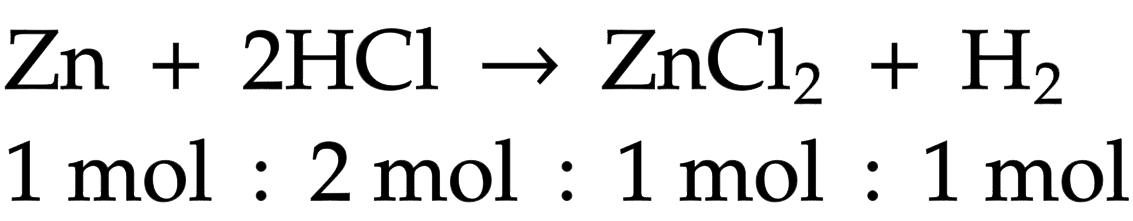
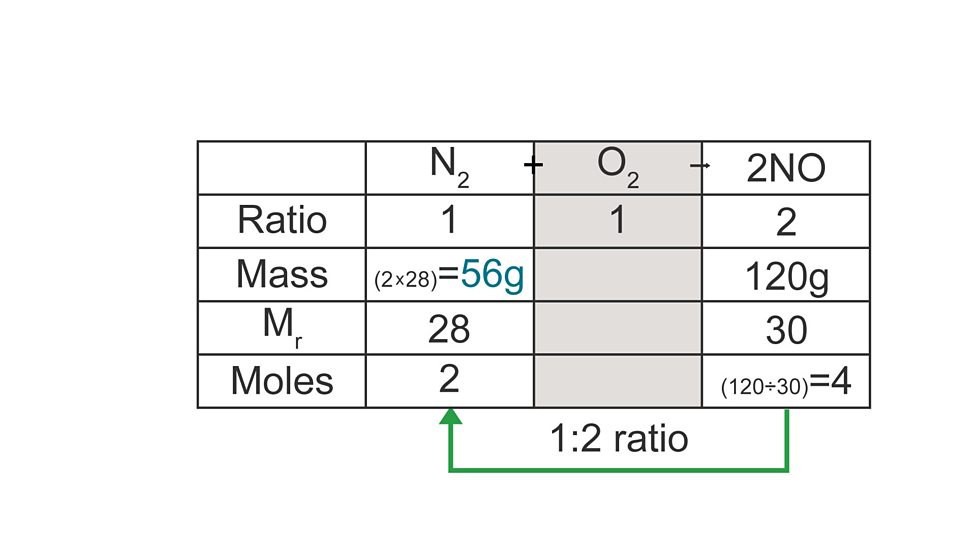
Calculating masses from moles
find moles of substance given in g using formula
use molar ratio to find mol of substance asked
convert moles into grams
Limiting reactant
reactant totally used up so it determines amount of product formed
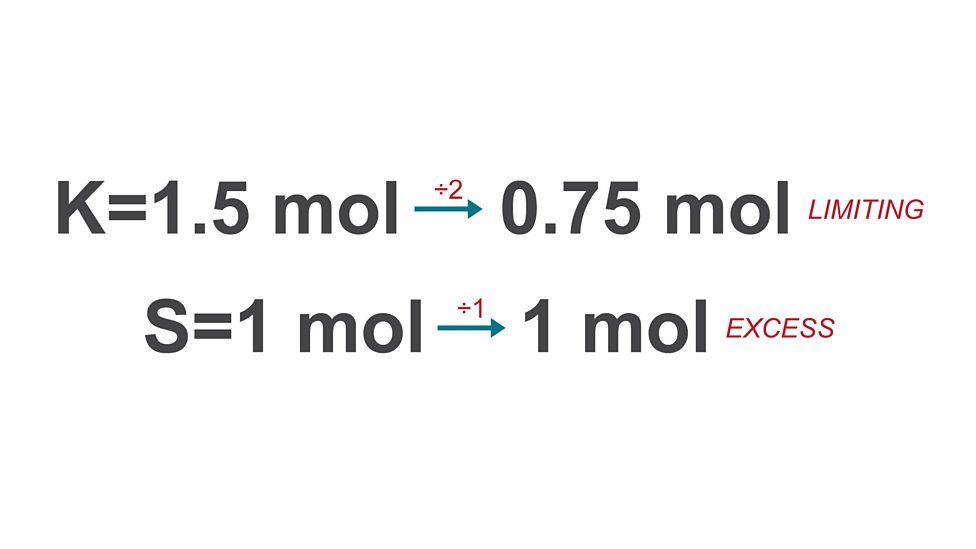
Excess
more than required to react with limiting reactant, left over
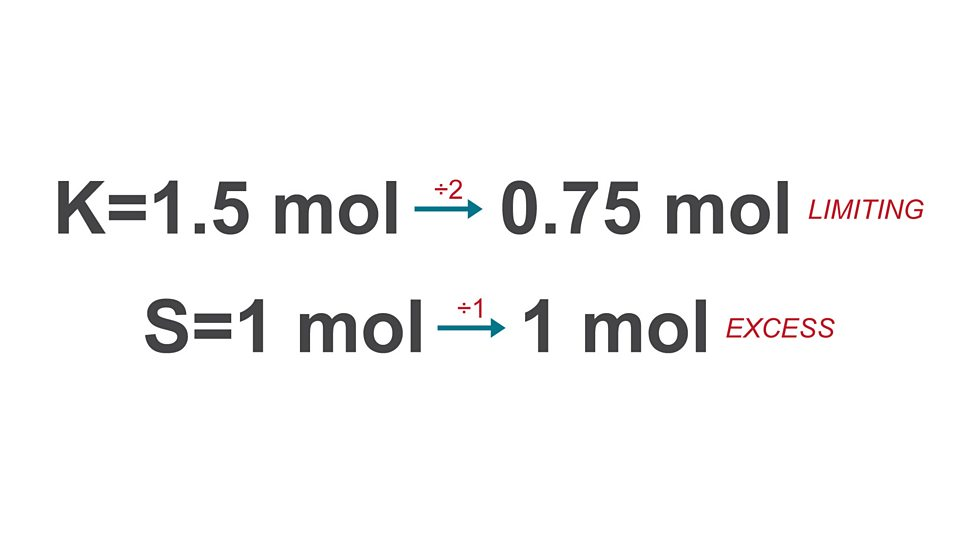
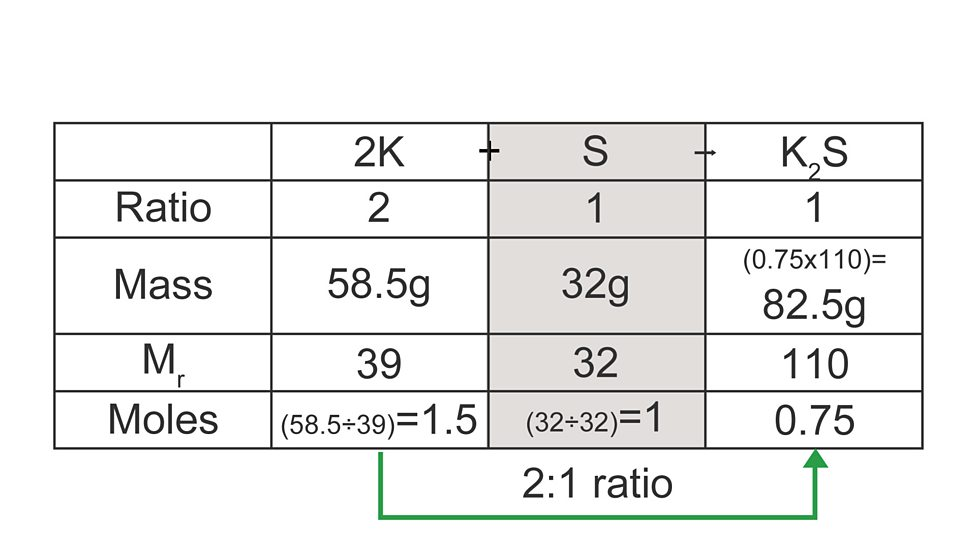
Calculating mass of substance formed
calculate moles for each reactant
divide mole values by molar ratio
use limiting reactant (smallest) to find final mass
Theoretical yield
maximum possible amount of product that can be made
Actual yield
amount of product made by carrying out the reaction
Reasons actual yield is less than theoretical
product is lost when removed from reaction mixture (stuck/ separation)
unwanted side reactions that use up reactants without forming desired product
reaction may be reversible so products reform reactants (⇌) and not going to completion
Percentage yield equation
actual yield/ theoretical yield x 100
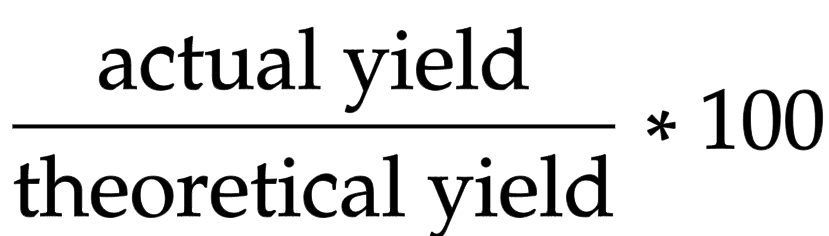
Why we use percentage yield
measure the efficiency of a reaction
Molecular formula
actual number of atoms of each element present in molecule
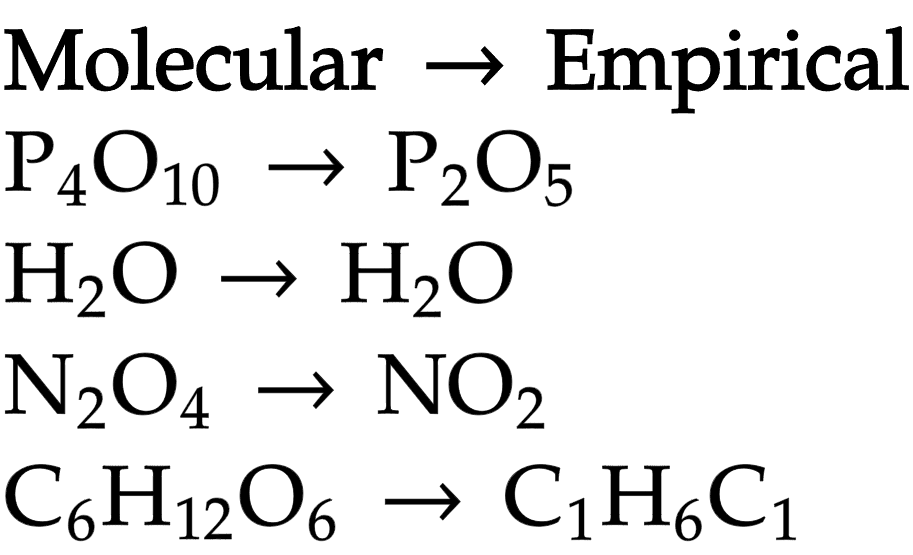
Empirical formula
simplest, whole number ratio of atoms of each element in compound
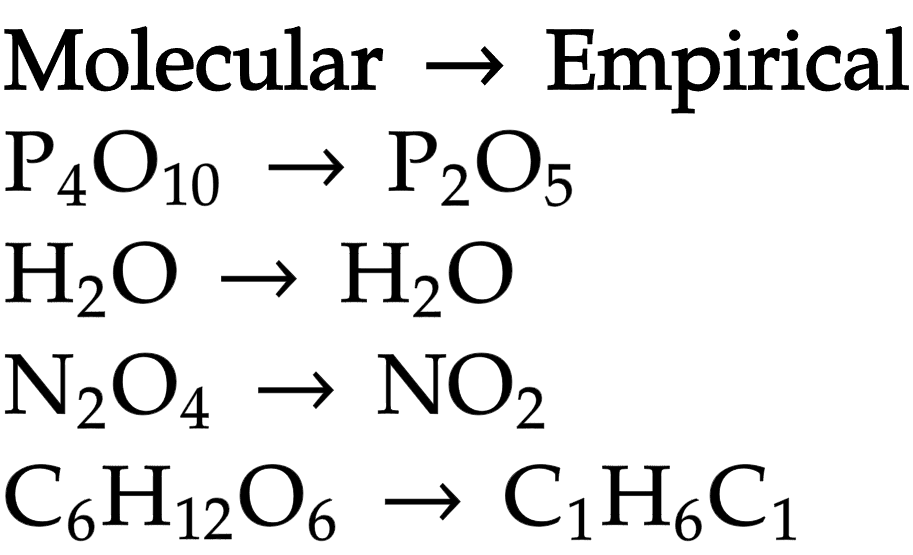
Calculating empirical formula of compound
heat compound to constant mass, metals increase (metal oxide)
find mass of each element by subracting
convert to moles using formula and simplify
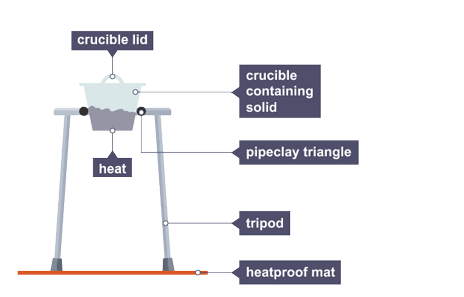
Heating to constant mass
heating and weighing, repeating this until two readings are the same
Water of crystallisation
water chemically bonded into the crystal structure
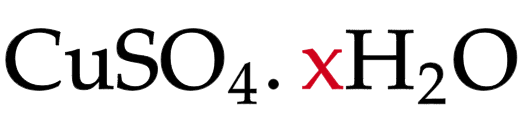
Hydrated
solid crystals contain water of crystallisation
Dehydration
removal of water of crystallisation
Anhydrous
does not contain water of crystallisation
Degree of hydration
number of moles of water of crystallisation chemically bonded in 1 mole of the compound
Calculating degree of water of crystallisation
find mass and Mr of each compound
calculate their moles
divide by smallest number of moles
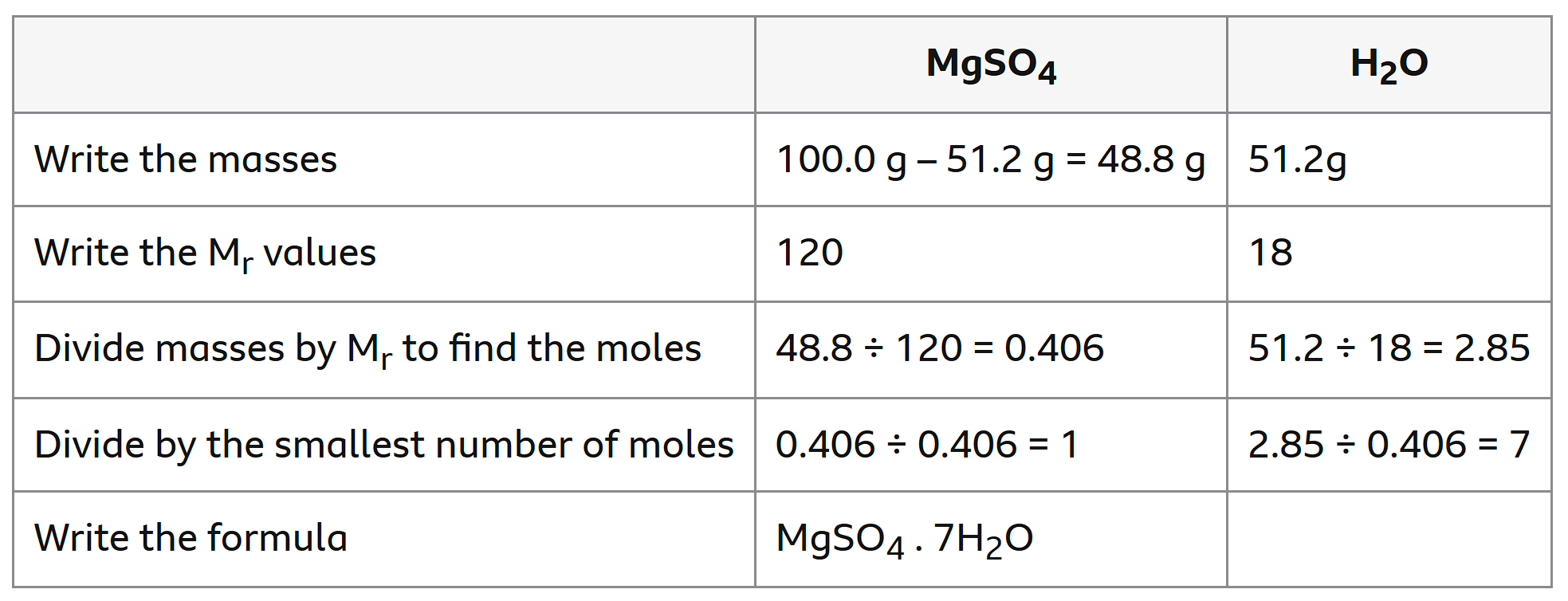
Determining percentage water of crystallisation
degree hydration * Mr of water/ Mr of compound * 100