chem hybridization w/ SPDF
1/97
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
98 Terms
4 bonds = what degree
109.5
4 bonds = what SPDF
Sp3
109.5 is what SPDF
Sp3
Sp3 = how many bonds
4
Sp3 = what degree
109.5
3 bonds= what degree
120
3 bonds= what spdf
sp2
120 is what SPDF
Sp2
Sp2= how many bonds
3
Sp = how many bonds
2
180 is what SPDF
Sp
2 bonds = what degree
180
2 bonds = what SPDF
Sp
what degree is p
90
90 = what SPDF
p
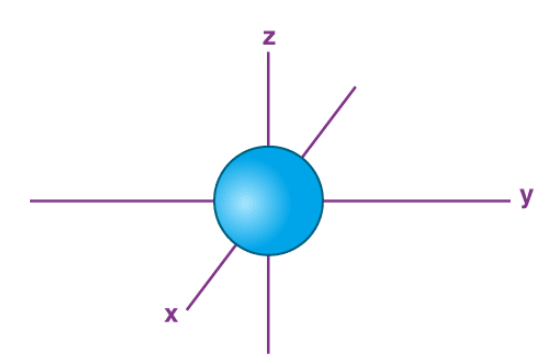
what orbital is this
s
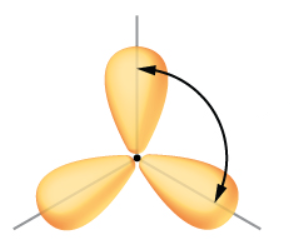
what orbital is this
Sp2
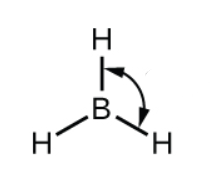
what SPDF is this
Sp2
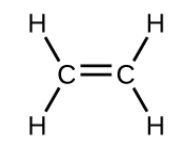
what SPDF are the central atoms
Sp2
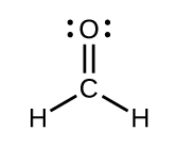
what SPDF is the central atom
Sp2
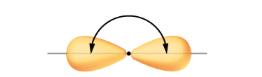
what SPDF is this
sp
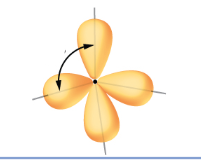
what SPDF is this
Sp3
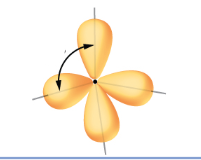
what degree is this
109.5

what degree is this
180

what degree is this
120
has at least one double bond
alkene
made of only single bonds
Alkane
has at least one triple bond
alkyne
alternating pattern of single and double bonds
aromatics
what type of newman projection is higher energy
eclipsed
what type of newman projection is lower energy
staggered
what type of newman projection is more stable
staggered
what type of newman projection is less stable
eclipsed
13CNMR signals represent
unique carbon atoms
IR signals represent
bonds
T/F: double bonds can rotate
false
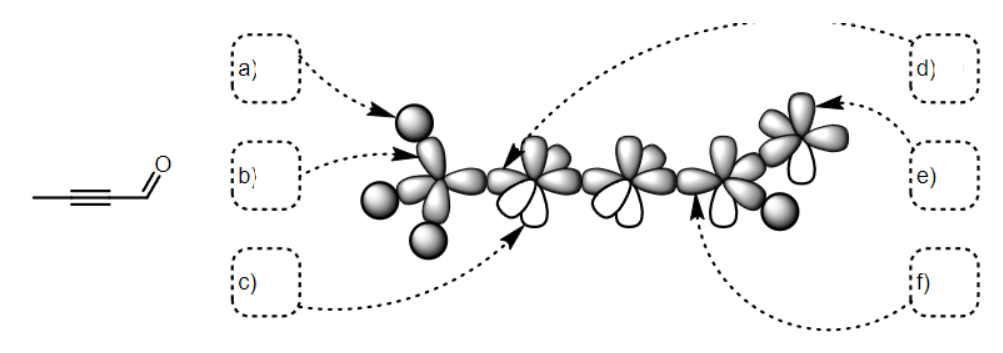
what SPDF is a
s
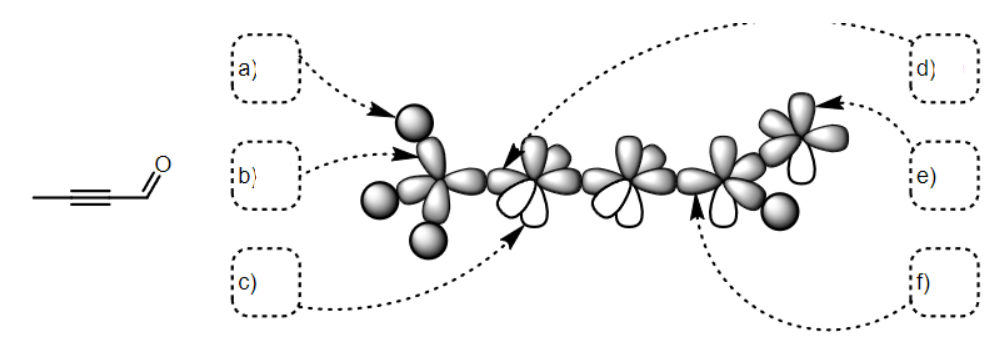
what SPDF is b
Sp3
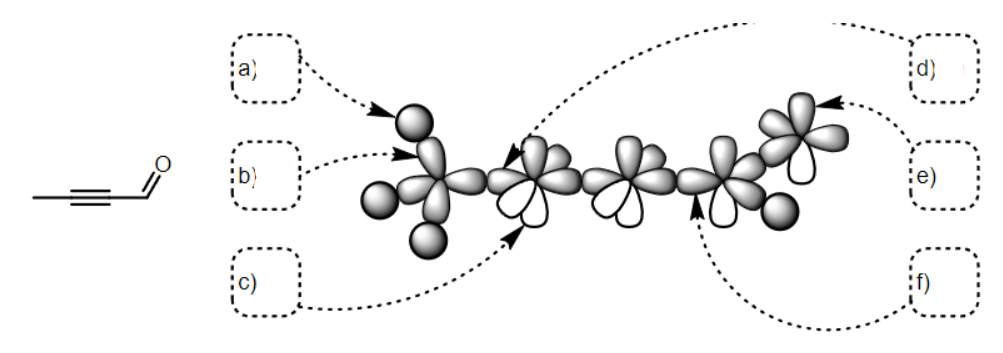
what SPDF is c
p
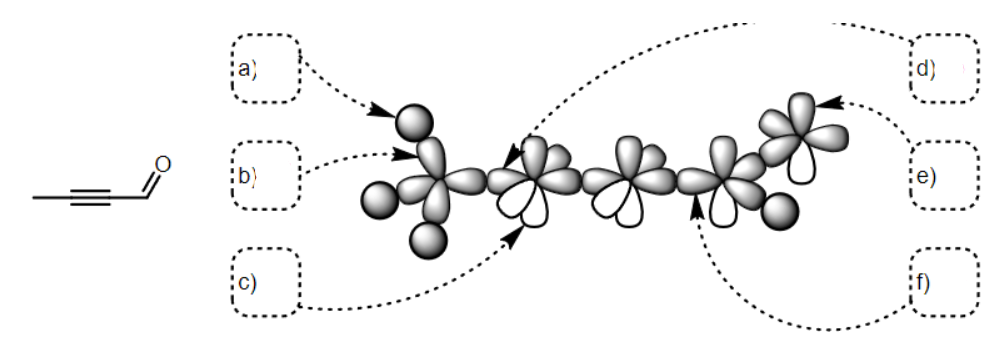
what SPDF is d
sp
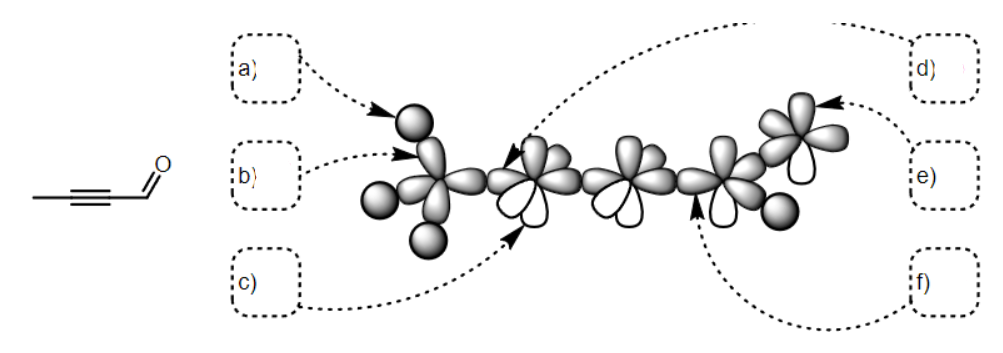
what SPDF is e
p
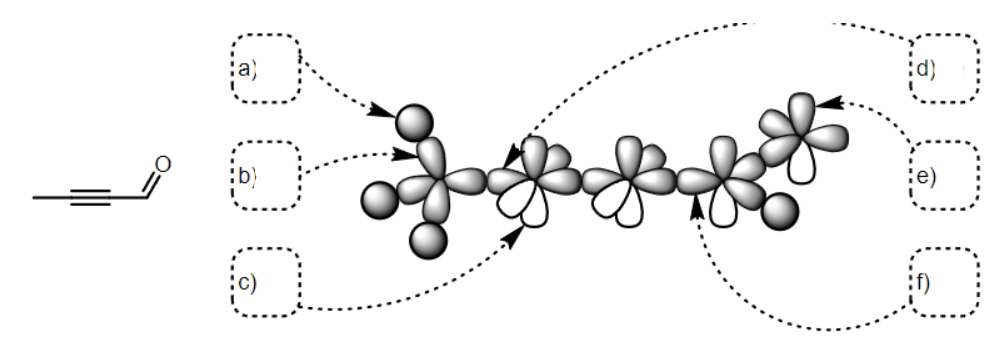
what SPDF is f
Sp2
isomers with the same formula but different connectivity are
constitutional isomers
isomers with the same formula but different spatial arrangement are
stereoisomers
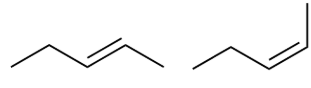
stereo or constitutional isomer
Stereoisomer
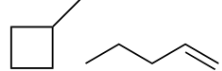
stereo or constitutional isomer
Constitutional isomer
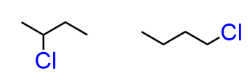
stereo or constitutional isomer
constitutional isomer
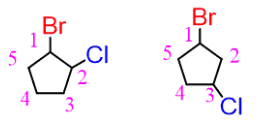
stereo/constitutional isomer or neither
constitutional isomer-
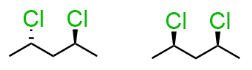
stereo/constitutional isomer, identical, or unrelated
stereoisomer-
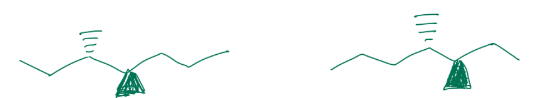
stereo/constitutional isomer, identical, or unrelated
identical

stereo/constitutional isomer, identical, or unrelated
stereoisomer!
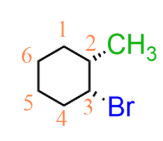
cis or trans
cis
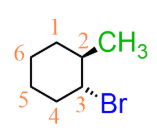
cis or trans
cis
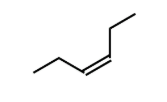
cis or trans
Trans
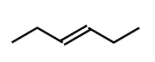
cis or trans
trans
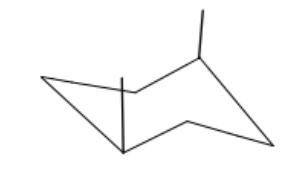
t/f: this is a chair conformation
false
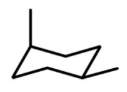
t/f: this is a chair conformation
true
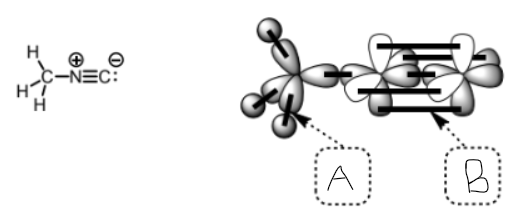
what type of bond is a
sigma
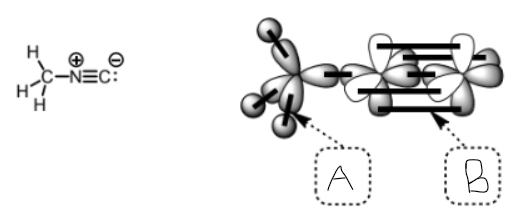
what type of bond is b
pi

what about the chair conformation makes these constitutional isomers?
the 1,4
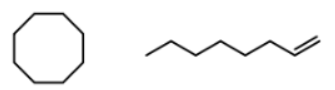
what does the double bond do to make these constitutional isomers
removes a hydrogen
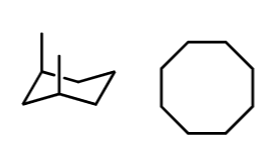
stereo/constitutional isomer, identical, or unrelated
constitutional isomer!
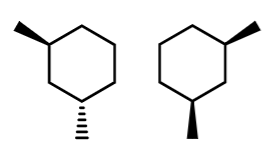
stereo/constitutional isomer, identical, or unrelated
stereoisomer~
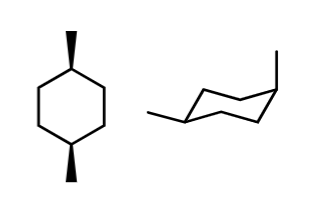
stereo/constitutional isomer, identical, or unrelated
Identical
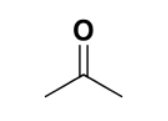
which functional group
carbonyl
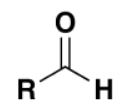
which functional group
aldehyde
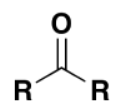
which functional group
ketone
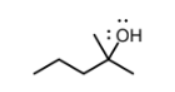
which functional group
alcohol
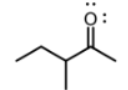
which functional group
ketone
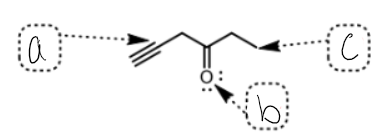
what SPDF is a
sp
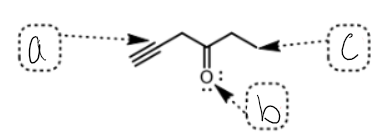
what SPDF is b
Sp2
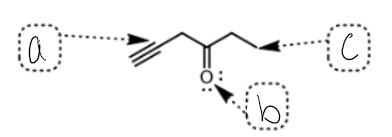
what SPDF is c
Sp3
what is the suffix for aldhydes
-al
when making a newman projection, do you need to include the implied hydrogens
yes
t/f: when doing a ring flip, you swap the ups and the down positions
False
t/f: when doing a ring flip, you swap the axial for equatorial (and vice versa)
True
in IR spectra to distinguish the Sp3 C-H bond from the Sp2 C-H bond, you should put a line at
3000
in IR spectra where does the fingerprint region end
1500
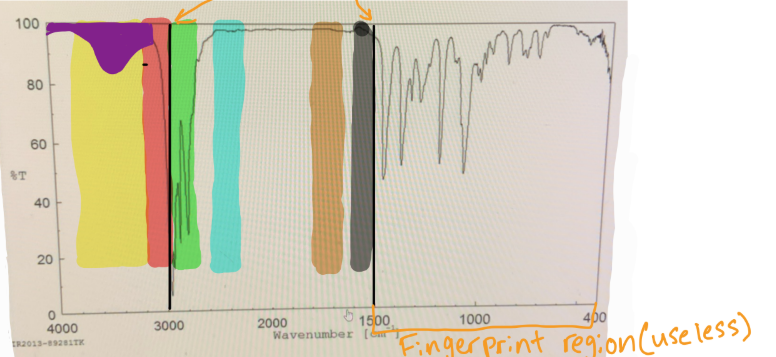
which color would you find O-H
yellow
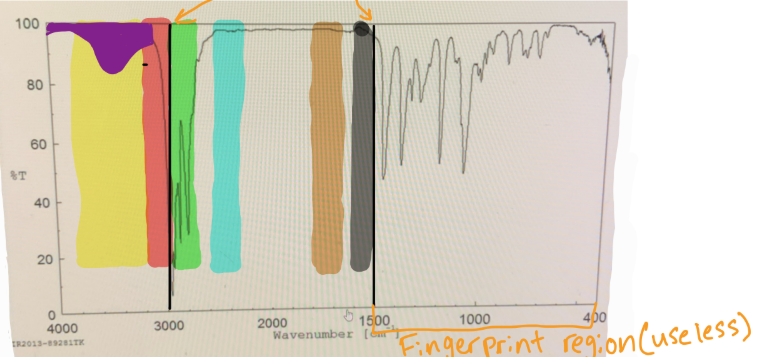
which color would you find N-H
yellow
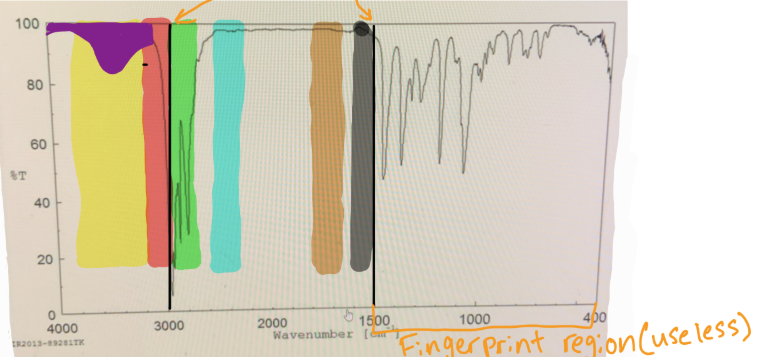
which color would you find Sp2 C-H
red
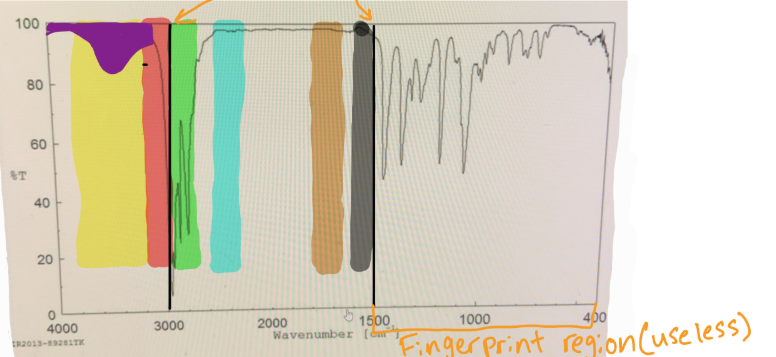
which color would you find Sp3 C-H
green
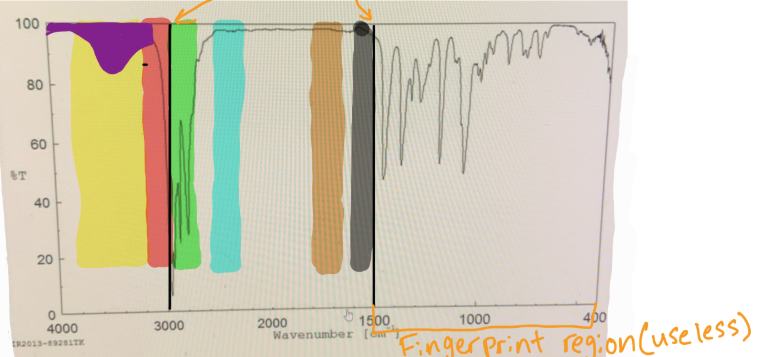
which color would you find C=C
black
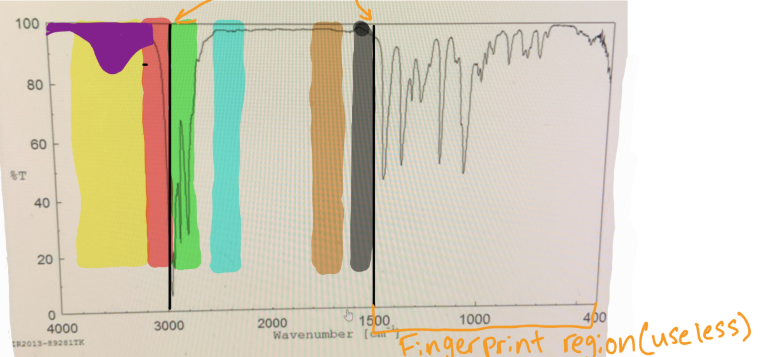
which color would you find C=O
brown
if something is “mound-shaped” in IR, what functional group is it (likely)
hydroxyl (OH)
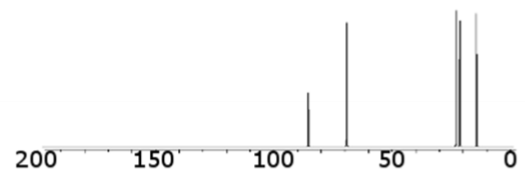
what type of bond is this consistent with (hydrocarbons)
triple
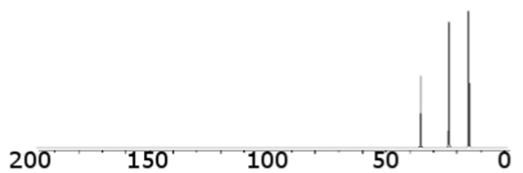
what type of bond is this consistent with (hydrocarbons)
single
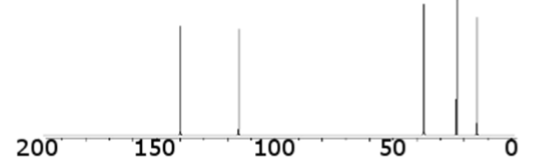
what type of bond is this consistent with (hydrocarbons)
double