Chapter 2
0.0(0)
0.0(0)
Card Sorting
1/116
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
117 Terms
1
New cards
Matter
Anything that has mass and takes up space
2
New cards
Mass
the amount of matter in an object
3
New cards
weight
the force of gravity on an object
4
New cards
Element
A pure substance made of only one kind of atom
5
New cards
Atom
is the smallest particle of an element that has the chemical characteristics of that element.
6
New cards
The four elements that make up about 96% of body weight are \________.
Carbon, hydrogen, oxygen and nitrogen.
Oxygen is the most abundant element in our body.
Oxygen is the most abundant element in our body.
7
New cards
Neutron
A subatomic particle that has no charge and that is found in the nucleus of an atom
8
New cards
Proton
A subatomic particle that has a positive charge and that is found in the nucleus of an atom
9
New cards
Electron
A subatomic particle that has a negative charge. The electrons are found in an electron cloud and are constantly orbiting the nucleus
10
New cards
what is the atomic number equal to?
number of protons and electrons (electrons \= protons)
11
New cards
mass number
is the number of protons plus the number of neutrons in each atom. For example, the mass number for carbon is 12 because it has 6 protons and 6 neutrons.
12
New cards
isotope
are two or more forms of the same element that have the same number of protons and electrons but a different number of neutrons. Thus, isotopes have the same atomic number but different mass numbers.
13
New cards
atomic mass
is the average mass of its naturally occurring isotopes, taking into account the relative abundance of each isotope.
14
New cards
Avogadro's number or 1 mole
number of representative particles in a mole, 6.02 X 10^23
15
New cards
mollar mass
The mass of 1 mole of a substance expressed in grams. Molar mass is a convenient way to determine the number of atoms in a sample of an element.
16
New cards
valence shell
The outermost energy shell of an atom, containing the valence electrons involved in the chemical reactions of that atom.
17
New cards
octet rule
States that atoms lose, gain or share electrons in order to acquire a full set of eight valence electrons
18
New cards
Cation
A positively charged ion
19
New cards
Aions
negatively charged ions
20
New cards
ionic bond
A chemical bond resulting from the attraction between oppositely charged ions.
A sodium atom (Na) loses an electron to become a smaller, positively charged ion, and a chlorine atom (Cl) gains an electron to become a larger, negatively charged ion. The attraction between the oppositely charged ions results in ionic bonding and the formation of sodium chloride.
A sodium atom (Na) loses an electron to become a smaller, positively charged ion, and a chlorine atom (Cl) gains an electron to become a larger, negatively charged ion. The attraction between the oppositely charged ions results in ionic bonding and the formation of sodium chloride.
21
New cards
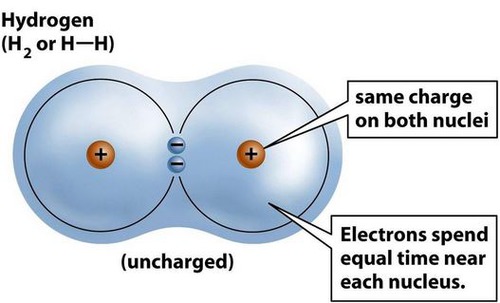
covalent bond
A chemical bond that involves sharing a pair of electrons between atoms in a molecule
Five examples of covalent bonds are hydrogen (H₂), oxygen (O₂), nitrogen (N₂), water (H₂O), and methane(CH₄)
Five examples of covalent bonds are hydrogen (H₂), oxygen (O₂), nitrogen (N₂), water (H₂O), and methane(CH₄)
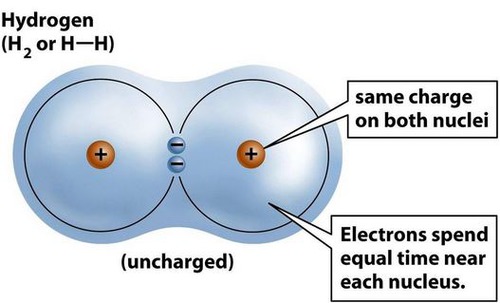
22
New cards
polar covalent bond
A covalent bond in which electrons are not shared equally
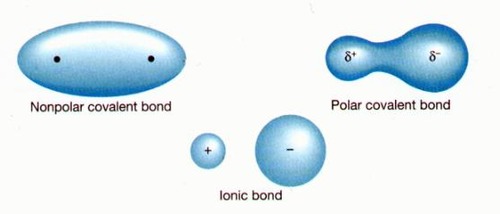
23
New cards
non polar covalent bond
a covalent bond in which the bonding electrons are shared equally by the bonded atoms, resulting in a balanced distribution of electrical charge.
24
New cards
single covalent bond
two atoms held together by sharing one pair of electrons
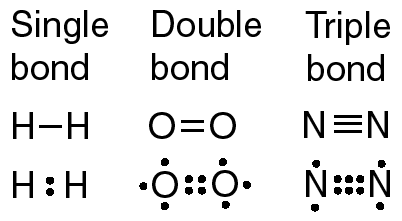
25
New cards
double covalent bond
a bond in which two atoms share two pairs of electrons
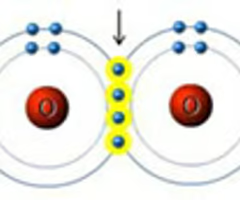
26
New cards
triple covalent bond
a covalent bond in which three pairs of electrons are shared by two atoms
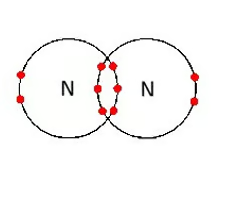
27
New cards
Molecule
two or more atoms chemically bonded together to form a structure.
28
New cards
Compound
is a substance resulting from the chemical combination of two or more different types of atoms. Water is a molecule that is also a compound because it is a combination of two different atoms, hydrogen and oxygen. But not all molecules are compounds. For example, a hydrogen molecule is not a compound because it does not consist of different types of atoms.
29
New cards
How do you find molar mass
adding up the atomic masses of its atoms (or ions). The term molecular mass is used for convenience for ionic compounds, even though they are not molecules. For example, the atomic mass of sodium is 22.99 and that of chloride is 35.45. The molecular mass of NaCl is therefore 58.44 (22.99 + 35.45).
30
New cards
intermolecular forces
the weak electrostatic attractions that exist between oppositely charged parts of molecules, or between ions and molecules. There is no exchange of electrons in intermolecular forces. This differs from other chemical bonds. Intermolecular forces are much weaker than the forces producing chemical bonding. Intermolecular forces include hydrogen bonds and the properties of solubility and dissociation.
31
New cards
hydrogen bond
A type of weak chemical bond formed when the slightly positive hydrogen atom of a polar covalent bond in one molecule is attracted to the slightly negative atom of a polar covalent bond in another molecule. For example, in water molecules (H2O), hydrogen is covalently bonded to the more electronegative oxygen atom. Therefore, hydrogen bonding arises in water molecules due to the dipole-dipole interactions between the hydrogen atom of one water molecule and the oxygen atom of another H2O molecule.
32
New cards
Solubility
is the ability of one substance to dissolve in another—for example, sugar dissolving in water. Charged substances, such as sodium chloride, and polar substances, such as glucose, readily dissolve in water, whereas nonpolar substances, such as oils, do not
33
New cards
Dissociation
the separation of ions that occurs when an ionic compound dissolves
34
New cards
Electrolyte
Cation or anion in solution that conducts an electric current.Electrolytes are essential for basic life functioning, such as maintaining electrical neutrality in cells and generating and conducting action potentials in the nerves and muscles. Significant electrolytes include sodium, potassium, chloride, magnesium, calcium, phosphate, and bicarbonates.
35
New cards
synthesis reaction
is when two or more reactants chemically combine to form a new and larger product. The collective term for synthesis reactions in the body is anabolism (an-AB-oh-lizm). These reactions produce the molecules characteristic of life, such as ATP, proteins, carbohydrates, lipids, and nucleic acids. The growth, maintenance, and repair of the body could not take place without anabolic reactions.
36
New cards
synthesis/dehydration reaction
Water is removed from the chemical reaction to synthesis or form biomolecules or polymers
37
New cards
Decompotion reaction
is the reverse of a synthesis reaction—a larger reactant is chemically broken down into two or more smaller products. The decomposition reactions occurring in the body are collectively called catabolism (kah-TAB-oh-lizm). They include the digestion of food molecules in the intestine and within cells, the breakdown of stored lipids, and the breakdown of foreign matter and microorganisms in certain blood cells that protect the body. All of the anabolic and catabolic reactions in the body are collectively defined as metabolism.
38
New cards
reversible reaction
is when the reaction can run in the opposite direction, so that the products are converted back to the original reactants.
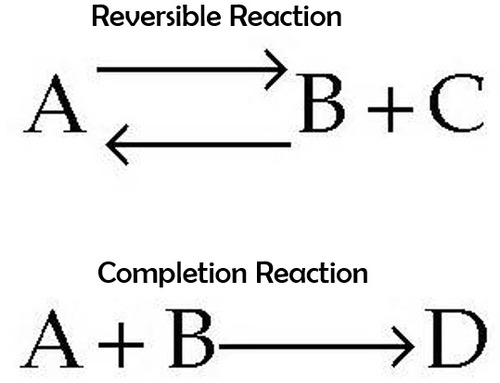
39
New cards
Oxidation
The loss of an electron by an atom
40
New cards
reduction
gain of electrons
41
New cards
energy
The ability to do work or cause change
42
New cards
potential energy
Energy in a chemical bond that is not being exerted or used to do work.
43
New cards
kinetic energy
Motion energy or energy that can do work.
44
New cards
mechanical energy
Kinetic or potential energy associated with the motion or position of an object
45
New cards
chemical energy
A form of potential energy that is stored in chemical bonds between atoms.
46
New cards
heat energy
a form of energy that is transferred by a difference in temperature
47
New cards
activation energy
Energy that must be added to molecules to initiate a reaction.
48
New cards
How do enzymes lower activation energy?
Enzymes generally lower activation energy by reducing the energy needed for reactants to come together and react. For example: Enzymes bring reactants together so they don't have to expend energy moving about until they collide at random.
49
New cards
catalyst
Substance that increases the rate at which a chemical reaction proceeds without being changed permanently.
50
New cards
inorganic chemistry
generally deals with substances that do not contain carbon, although a more rigorous definition is the lack of carbon-hydrogen bonds.
51
New cards
organic chemistry
is the study of carbon-containing substances, with a few exceptions. For example, carbon monoxide (CO), CO2, and bicarbonate ions (HCO3−) are several important inorganic substances that contain carbon but lack C—H bonds.
52
New cards
five important inorganic molecules we need for life
(1) O2 we breathe, (2) CO2 we exhale, (3) water essential for life, (4) calcium phosphate that makes up our bones, and (5) metals required for protein functions, ranging from iron in blood gas transport to zinc in alcohol detoxification.
53
New cards
What are the 4 important functions that water performs in the body?
stabilize body temp, protection, chemical reactions, and mixing medium
54
New cards
Choesion
attraction of water to another water molecule. Examples of cohesion are the surface tension exhibited when water bulges over the top of a full glass without spilling over and when beads of water form on the skin.
55
New cards
Adhesion
attraction of water to other molecules. An example of adhesion is the surface tension that draws water across a glass plate and holds a bead of water to the skin before it falls to the ground. For both cohesion and adhesion, the attractive force is hydrogen bonds. The combination of cohesion and adhesion helps hold cells together and move fluids through the body.
56
New cards
Mixture
is a combination of two or more substances physically blended together, but not chemically combined
57
New cards
Solution
is any mixture in which the substances are uniformly distributed. Solutions can be liquid, gas, or solid. For example, a salt solution consists of salt dissolved in water, air is a solution containing a variety of gases, and wax is a solid solution composed of several fatty substances.
58
New cards
Solute
A substance that is dissolved in a solution.
59
New cards
Solvent
the substance in which the solute dissolves
60
New cards
Suspension
Liquid through which a solid is dispersed and from which the solid separates unless the liquid is kept in motion.
a mixture containing materials that separate from each other unless they are continually, physically blended together. Blood is a suspension—that is, red blood cells are suspended in a liquid called plasma. As long as the red blood cells and plasma are mixed together as they pass through blood vessels, the red blood cells remain suspended in the plasma. However, if the blood is allowed to sit in a container, the red blood cells and plasma separate from each other.
a mixture containing materials that separate from each other unless they are continually, physically blended together. Blood is a suspension—that is, red blood cells are suspended in a liquid called plasma. As long as the red blood cells and plasma are mixed together as they pass through blood vessels, the red blood cells remain suspended in the plasma. However, if the blood is allowed to sit in a container, the red blood cells and plasma separate from each other.
61
New cards
Colloid
a mixture in which a dispersed substance or particle is unevenly distributed throughout the mixture. Unlike a suspension, the dispersed particles are small enough that they do not settle out. Proteins are common dispersed particles; proteins and water form colloids. For instance, the plasma portion of blood and the liquid interior of cells are colloids containing many important proteins.
62
New cards
Acid
Molecule that is a proton donor; any substance that releases hydrogen ions (H+).
63
New cards
base
Molecule that is a proton acceptor; any substance that binds to hydrogen ions. Lower part or bottom of a structure; the base of the heart is the flat portion directed posteriorly and superiorly; veins and arteries project into and out of the base, respectively.
64
New cards
What is a molecule called that releases H+?
Acid
65
New cards
What is meant by a strong or weak acid/base?
Any acid that dissociates 100% into ions is called a strong acid. If it does not dissociate 100%, it is a weak acid.
66
New cards
pH scale
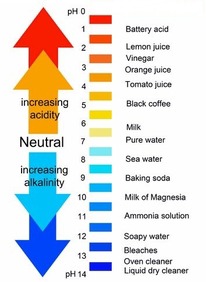
67
New cards
Acidosis
Acidosis results if blood pH drops below 7.35. In this case, the nervous system becomes depressed and the individual may become disoriented and possibly comatose.
68
New cards
Alkalosis
Alkalosis results if blood pH rises above 7.45. In this case, the nervous system becomes overexcitable, and the individual may become extremely nervous or have convulsions. Both acidosis and alkalosis can be fatal.
69
New cards
Normal pH of blood
The normal pH range for human blood is 7.35 to 7.45
70
New cards
Salt
A salt is a compound consisting of a cation other than H+ and an anion other than OH−.
71
New cards
How are salts formed?
Salts are formed by the interaction of an acid and a base in which the H+ of the acid are replaced by the positive ions of the base. For example, in a solution in which hydrochloric acid (HCl) reacts with the base sodium hydroxide (NaOH), the salt sodium chloride (NaCl) is formed:
72
New cards
Buffer
chemicals that resist changes in pH when either acids or bases are added to a solution. For example, when an acid is added to a buffered solution, the buffer binds to the H+, preventing these ions from causing a decrease in the pH of the solution
73
New cards
What are the four essential molecules to living things?
carbohydrates, lipids, proteins, and nucleic acids
74
New cards
Carbohydrates
organic molecules composed primarily of carbon, hydrogen, and oxygen atoms and range in size from small to very large.
75
New cards
What three roles do carbohydrates play in the human body?
(1) They are parts of other organic molecules, (2) they are broken down to provide energy, and (3) when undigested they provide bulk in feces.
76
New cards
Monosaccharides
Simple sugar carbohydrate that cannot form any simpler sugar by hydrolysis.
Monosaccharides commonly contain 3 carbons (trioses), 4 carbons (tetroses), 5 carbons (pentoses), or 6 carbons (hexoses).
Monosaccharides commonly contain 3 carbons (trioses), 4 carbons (tetroses), 5 carbons (pentoses), or 6 carbons (hexoses).
77
New cards
Disaccharide
Condensation product of two monosaccharides by the elimination of water.
For example, glucose and fructose combine to form a disaccharide called sucrose (table sugar) plus a molecule of water. In addition to sucrose, two other disaccharides important to humans are lactose and maltose.
For example, glucose and fructose combine to form a disaccharide called sucrose (table sugar) plus a molecule of water. In addition to sucrose, two other disaccharides important to humans are lactose and maltose.
78
New cards
Polysaccharides
Carbohydrate containing a large number of monosaccharide molecules.
Three important polysaccharides are glycogen, starch, and cellulose.
Three important polysaccharides are glycogen, starch, and cellulose.
79
New cards
Isomers
Molecules having the same number and types of atoms but differing in their three-dimensional arrangement.
Common 6-carbon sugars, such as glucose, fructose, and galactose, are isomers
Common 6-carbon sugars, such as glucose, fructose, and galactose, are isomers
80
New cards
Glycogen
Glycogen, or animal starch, is a multibranched polysaccharide composed of many glucose molecules. Glycogen is the main storage form of glucose in humans.
81
New cards
Starch/cellulose
Starch and cellulose are two important polysaccharides found in plants. Like glycogen, both are composed of long chains of glucose. Starch is an energy-storage molecule in plants, similar to the role of glycogen in animals. Cellulose is an important structural component of plant cell walls. When humans ingest plants, the starch can be broken down and used as an energy source. Humans, however, do not have the digestive enzymes necessary to break down cellulose. Cellulose is eliminated in the feces, where it provides bulk. Clinically, the presence of cellulose (fiber) in our diet is important for regularity of bowel movements and has been reported to help reduce cholesterol and control blood sugar levels.
82
New cards
Lipids
Substance composed principally of carbon, oxygen, and hydrogen; contains a lower ratio of oxygen to carbon and is less polar than carbohydrates; generally soluble in nonpolar solvents.
83
New cards
Lipids main role in body
They (1) provide protection and insulation, (2) help regulate many physiological processes, (3) form plasma membranes, and (4) act as major energy-storage molecules.
84
New cards
What is a lipid made up of?
Like carbohydrates, lipids are composed principally of carbon, hydrogen, and oxygen, but lipids have a lower ratio of oxygen to carbon than do carbohydrates. This makes them less polar.
85
New cards
What can lipids dissolve in?
lipids can be readily dissolved in nonpolar organic solvents, such as alcohol or acetone, but they are relatively insoluble in water. Some lipids also contain small amounts of other elements, such as phosphorus and nitrogen, which can aid solubility in water.
86
New cards
The five major classes of lipids are
(1) fats, which are mostly triglycerides; (2) phospholipids; (3) eicosanoids; (4) steroids; and (5) fat-soluble vitamins.
87
New cards
Adipose
Adipose tissue is a specialized connective tissue consisting of lipid-rich cells called adipocytes. As it comprises about 20-25% of total body weight in healthy individuals, the main function of adipose tissue is to store energy in the form of lipids (fat).
88
New cards
Triglycerides
Three-carbon glycerol molecule with a fatty acid attached to each carbon; constitutes approximately 95% of the fats in the human body. Also called triacylglycerol.
Triglycerides consist of two different types of building blocks: (1) one glycerol and (2) three fatty acids.
Triglycerides consist of two different types of building blocks: (1) one glycerol and (2) three fatty acids.
89
New cards
saturated fatty acid
Fatty acid in which the carbon chain contains only single bonds between carbon atoms.
Sources of saturated fats include beef, pork, whole milk, cheese, butter, eggs, coconut oil, and palm oil.
Sources of saturated fats include beef, pork, whole milk, cheese, butter, eggs, coconut oil, and palm oil.
90
New cards
unsaturated fatty acid
Carbon chain of a fatty acid that possesses one or more double or triple bonds.
Unsaturated fats:
Olive, peanut, and canola oils.
Avocados.
Nuts such as almonds, hazelnuts, and pecans.
Seeds such as pumpkin and sesame seeds.
Unsaturated fats:
Olive, peanut, and canola oils.
Avocados.
Nuts such as almonds, hazelnuts, and pecans.
Seeds such as pumpkin and sesame seeds.
91
New cards
monounsaturated fats
such as olive and peanut oils, have one double covalent bond between carbon atoms.
92
New cards
polyunsaturated fats
such as safflower, sunflower, corn, and fish oils, have two or more double covalent bonds between carbon atoms. Unsaturated fats are the best type of fats in the diet because, unlike saturated fats, they do not contribute to the development of cardiovascular disease.
93
New cards
trans fat
unsaturated fats that have been chemically altered by the addition of hydrogen. The process makes the fats more saturated and hence more solid and stable (longer shelf life). However, the double covalent bonds that do not become saturated are changed from the usual cis configuration (H on the same side of the double bond) to a trans configuration (H on different sides). This change in structure makes the consumption of trans fats an even greater factor than saturated fats in the risk for cardiovascular disease.
94
New cards
Choesterol
is a steroid hormone. It is the backbone of all your hormones of your body. Without cholesterol you do not make things like estrogen and testosterone.
95
New cards
Protein
Macromolecule consisting of long sequences of amino acids linked together by peptide bonds. contain carbon, hydrogen, oxygen, and nitrogen bound together by covalent bonds. Most proteins also contain some sulfur. In addition, some proteins contain small amounts of phosphorus, iron, and iodine.
96
New cards
five important functions of proteins
(1) regulate body processes, (2) act as transportation molecules, (3) provide protection, (4) help muscles contract, and (5) provide structure and energy.
97
New cards
Basic building blocks of proteins
The basic building blocks for proteins are the 20 amino acid molecules. Each amino acid has an amine group (—NH2), a carboxyl group (—COOH), a hydrogen atom, and a side chain designated by the symbol R attached to the same carbon atom. The side chain can be a variety of chemical structures, and the differences in the side chains make the amino acids different from one another.
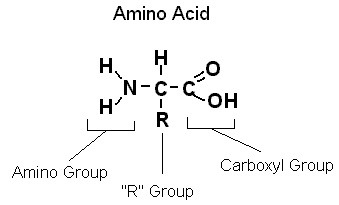
98
New cards
peptide bond
Covalent bonds formed between amino acid molecules during protein synthesis
99
New cards
Dipeptide
two amino acids bound together by a peptide bond
100
New cards
tripeptide
three amino acids covalently bound together by peptide bonds