Chapter 12 - Alkanes
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
18 Terms
alkane
saturated hydrocarbon only containing single C-C and C-H bonds
general formula and shape
CnH2n+2
shape = tetrahedral = 109.5
sigma (σ) bonding in alkanes
-a covalent bond where electron orbitals directly overlap between bonding atoms
-contains a pair of electrons - 1 electron from each orbital of each atom
-there is free rotation of the carbon atoms
why are alkanes VERY UNREACTIVE?
-has high bond enthalpy
-has very low polarity of sigma bonds
so are non-polar as carbon and hydrogen have similar electronegativities
how are C-C and C-H sigma bonds formed?
-the 2p orbital and 2s orbital in carbon contain the 4 outer electrons
-these electrons merge together to form 4 new orbitals called sp3 orbitals - 1 sp3 orbital from each carbon atom then overlaps to form the C-C sigma bond
-the 3 remaining sp3 orbitals from each atom overlap with the 1s orbital of the 3 hydrogen atoms to form 3 C-H sigma bonds
BOILING POINTS of alkanes: why do alkanes go from liquid to gaseous when heated?
there are London forces between the alkane chains which hold the molecules close together in liquid state but as they are weak attractive forces, they are broken when heated causing the molecules to move far apart and so the chain separates
why does the boiling point increase as the carbon chain length increases?
the surface area of the molecules increases so the number of contact points between adjacent molecules increases so increases the number and strength of London forces so more energy needed to break
why do BRANCHED alkanes have lower boiling points than UNBRANCHED alkanes?
branched have fewer points of contact as the molecules cannot fit close together and makes the molecules go further apart due to the branches so number of London forces decrease so less energy is needed
why are alkanes good fuels?
-have strong, non-polar C-C and C-H bonds = makes alkanes stable
-C-C and C-H bonds have high bond enthalpies = lots of heat energy is required
-therefore when these bonds are broken during combustion, large amounts of heat energy is released (very exothermic)
COMPLETE combustion
-in excess oxygen
alkane + oxygen → carbon dioxide + water
INCOMPLETE combustion
-oxygen is limited
alkane + oxygen → carbon monoxide + water
-concentration of oxygen is extremely low
alkane + oxygen → carbon (soot) + water
-mixture of both
alkane + oxygen → carbon monoxide + carbon (soot) + water
free radical substitution
happens in the presence of UV light and forms a haloalkane (desired product) and hydrogen halide (by-product)

radical
a species with an unpaired electron
homolytic fission
when each bonding atom receives 1 electron from a bonding pair of electrons causing covalent bond to break and 2 radicals to form
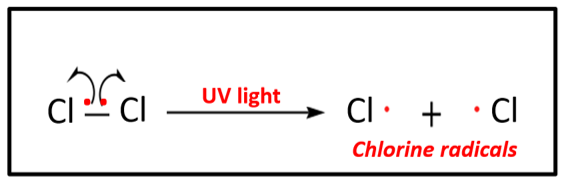
3 STEPS of free radical substitution 1) initiation
the reaction is started when the covalent bond in a halogen is broken by homolytic fission and forms free-radicals
Cl2 → 2Cl . + UV light
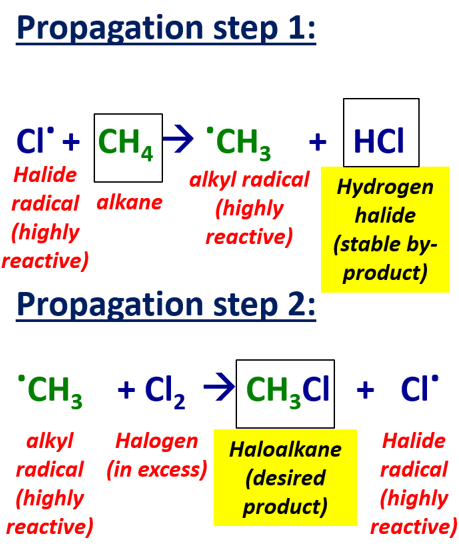
2) propagation
-chain reaction where a free radical reacts with an alkane and more radicals are formed so process is repeated
step 1 = a halide radical reacts with hydrogen atom in C-H bond of alkane which forms a hydrogen halide and an alkyl radical
Cl . + CH4 → CH3 . + HCl
step 2 = the alkyl radical remaining reacts with a halogen forming a haloalkane and a halide radical
CH3 . + Cl2 → Cl . + CH3Cl
3) termination
2 radicals collide and react to form an unreactive substance so stops the chain reaction
Cl . + Cl . → Cl2
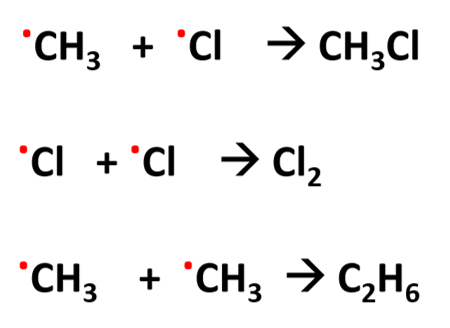
LIMITATIONS of free radical substitution
-very unpredictable and difficult to control
-further substitutions
-substitutions at different positions
-so mixture of products formed