PBI288 - Final review
1/118
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
119 Terms
Transduction
Process of changing stimulus ( energy, force, chemical...) from the environment into an electrochemical signal for the nervous system; Requires appropriate receptors & biochemical process in receptors that activate neurons
Sensory receptor
Specialized type of cell that responds to environmental stimulation (photoreceptors, cutaneous touch receptors)
Cell membrane receptor
Specialized type of protein on a cell that responds to biochemical activation (ionotropic receptors, metabotropic receptors)
Receptor Potential
Slow, moderate change in electrical state of a cell ( passive change, EPSP or IPSP)
Action Potential
Rapid, large change in electrical state of a cell (“all or none“ event, active propagation)
Summarize how activation of photoreceptors in the retina is transmitted to the lateral geniculate nucleus (LGN) in the thalamus
Photoreceptors in retina activated by light
Signals transmitted from photoreceptors to bipolar cells then ganglion cells
Ganglion cells form optic nerve, carry signals to brain
Signals reach LGN in thalamus
Within LGN signals undergo processing
Processed signals relayed to primary visual cortex in occipital lobe
Compare/contrast two types of photoreceptors in the human eye
Cones: most prevalent in central retina; found in fovea; sensitive to moderate-high levels of light; provide info about hue; provide excellent acuity.
Rods: most prevalent in peripheral retina; not found in fovea; sensitive in low levels of light; provide only monochromatic info; provide poor acuity.
Describe how differences in light-sensitive receptor proteins contribute to colour blindness
Colour blindness results from malfunction/absence of one or more types of cone cells in retina
Differences in opsin genes, which code for cone cell proteins, contribute to colour blindness
Variations in opsin can lead to: missing/non-functioning red, green or blue cone cells & reduced sensitivity/altered spectral sensitivity of cone cells
Types of colour blindness: red-green (protanopia & deuteranopia)& blue-yellow (tritanopia)
Affected individuals may have trouble distinguishing certain colours/perceive them differently
Colour blindness can impact daily tasks such as interpreting traffic signals/selecting ripe fruits
Summarize how information from left & right visual fields is directed to the right & left brain hemispheres
Each eye receives input from both left & right visual fields
Optic nerves from both eyes meet at optic chiasm (some fibers cross to opposite side, some continue straight)
Fibers carrying information from nasal (inner) retina of each eye cross at optic chiasm to opposite side of brain
Fibers carrying information from temporal (outer) retina of each eye stay straight on to same side of brain
Visual information from left visual field of both eyes processed in right hemisphere of brain & visual information from right visual field of both eyes processed in left hemisphere of brain
Arrangement ensures that each hemisphere processes information from both visual fields, enabling integrated perception of entire visual field in each hemisphere
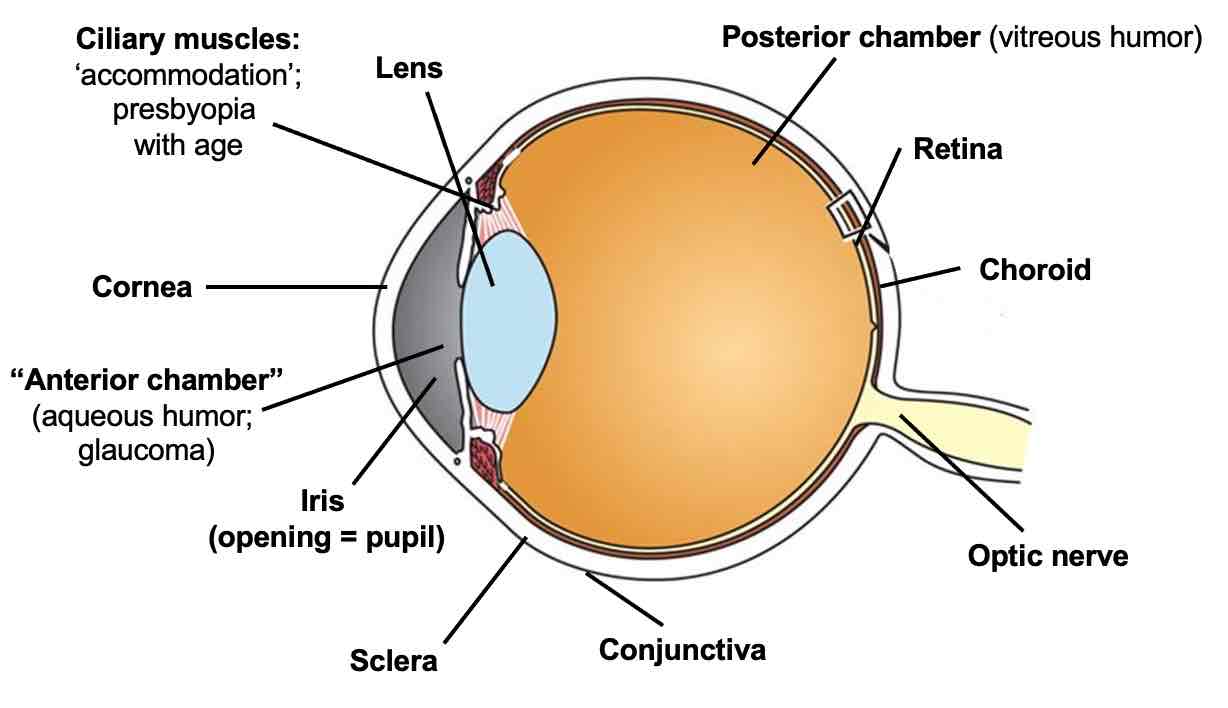
Anatomy of the eye
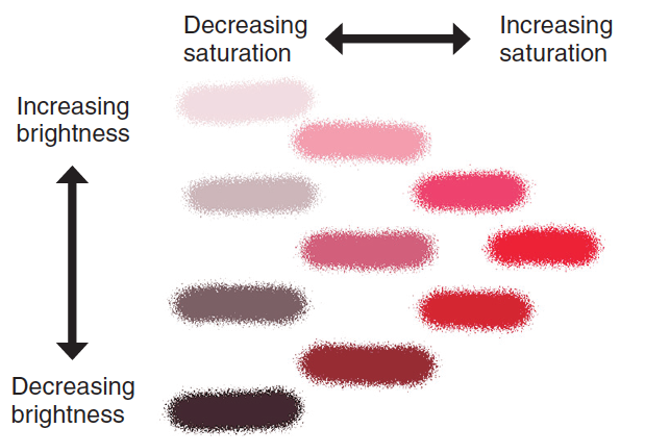
Properties of light
Hue → specific/dominant wavelength (colour)
Brightness → intensity of light (amount of light present)
Saturation → “purity“ of light, richness of colour (just one wavelength vs many other wavelengths)
Binocular vision
Each eye has slightly different view of a given object, because of small difference between eyes
Merging images helps us perceive depth
Rhodopsin
Photopigment in rods that responds to light
Opsin
Photopigment in cones
Photopigment
Made up of opsin (protein) & retinal (lipid)
Light strikes photopigment molecules & splits it into opsin & retinal components
“Dark current”
In darkness, photoreceptors release glutamate at synapses with bipolar neurons
Receptive fields
Represents part of visual field that it responds to (changes its firing rate)
Constituency of cells that provide info to retinal ganglion cell MP (think of it as government)
Organization of receptive fields
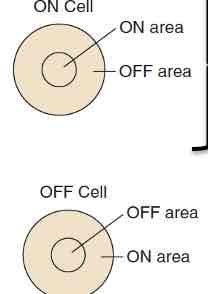
On-centre/off-surround cells: likes light in middle of its receptive field
Off-centre/on-surround cells: likes light around perimeter of its receptive field
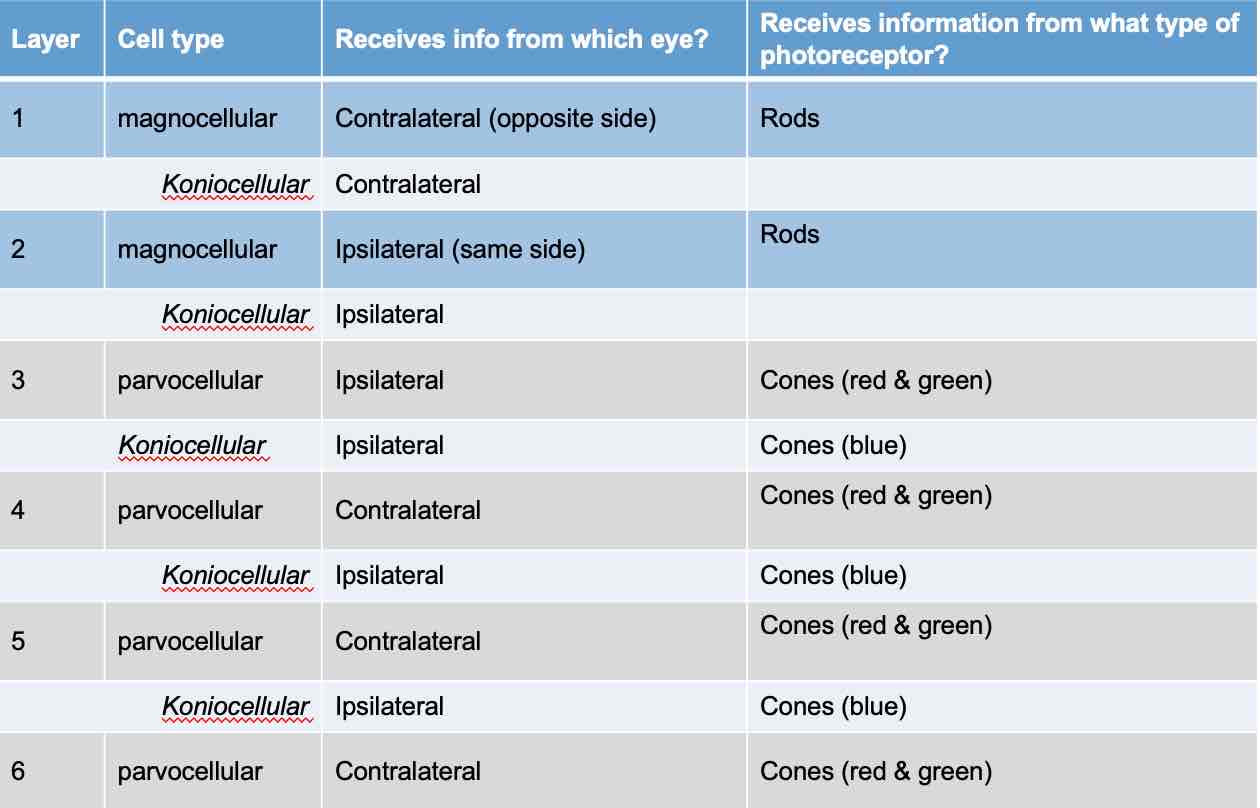
Lateral Geniculate Nucleus
Bilateral, multilayered structure (6 )
Each layer receives specific types of info from retina
Layers distinguished by cell size ( magnocellular = large, parvocellular =small, koniocellular = tiny)
Optic chiasm
Splitter
Axons coming from medial/nasal part of retina cross to opposite hemisphere at optic chiasm
Axons coming from lateral/peripheral part of retina do not cross
Info from left visual field is directed to right LGN (& right visual cortex)
From retina to brain
RGC axons leave eye (optic nerve)
Optic chiasm
1st synapse in LGN in thalamus
2nd synapse in primary visual cortex (V1)
V1: organization
Striate cortex
6 layers: inputs from LGN enter at layer 4 of V1, inputs from ipsilateral & contralateral eyes kept separate in layer 4
Organized into modules or small ‘columns‘ of cortex
V1: representation of visual field
Retinotopic map preserved in V1 & fovea processed by proportionally more cells than in any other area of retina
List the characteristics of visual information processed by cells in V1 (primary visual cortex)
Orientation: most neurons in V1 sensitive to specific orientations of light-dark information in its receptive field, sensitive= more neuron firing when light/dark boundary oriented at specific angle
Spatial frequency: changes in brightness, refers to # of times area of visual information shifts (light→dark→light→dark), high frequency information= lots of small details & large objects with clear edges/contours, low frequency information=visual scenes with large objects & blurry/ not too much detail
Retinal disparity: binocular cells become very active when image of an object appears on slightly different areas of left & right retinas (difference in location between eyes), crossed-disparity=objects close to us, uncrossed-disparity= objects far away from us
Colour: third stage of colour processing occurs in V1→ colour-sensitive cells in blobs
Movement
V1: modules
Each module contains a tiny region of all 6 layers of V1 ( 0.5mm diameter)
Each module ‘sees‘ information from tiny part of the retina
Cells within single module specialize for orientation (180°), left-right eye (binocular & monocular preference, disparity gives info about distance)
4 colour blobs per module
Movement
Spatial frequency
Extrastriate visual cortex
Areas of visual cortex outside V1 (visual association cortex,~ 25% of human cerebral cortex)
Each area specialized for aspects of visual information
Receive info from V1→process →pass on information to higher areas of extrastriate cortex ( area V4→colour processing, area V5→movement)
V2 & V3 cells→similar properties to V1 (retinotropic organization, cells selective for angle/direction of motion, cortical magnification, most are binocular)
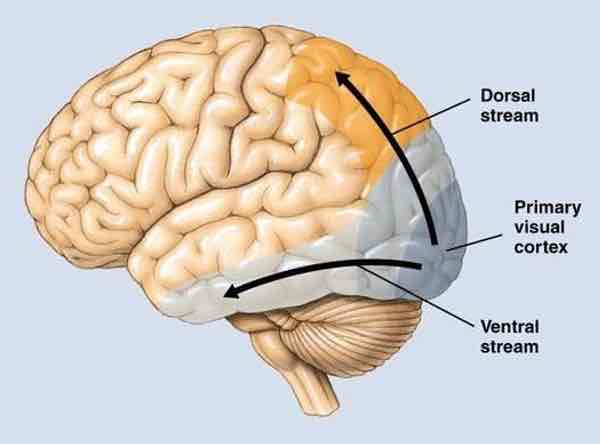
Ventral vs dorsal stream
Visual processing
~ 25-30% of human cerebral cortex involved in visual processing
Ventral stream (extrastriate visual cortex)
”what“ pathway
Allows for object recognition
P pathway, some K pathway (colour, texture, detail)
Signals forwarded to parts of inferior temporal lobes
Vision for recognition (1. Cells in each region respond to more complex stimuli 2. Cases of IT cortex damage→agnosias 3. Very large receptive fields)
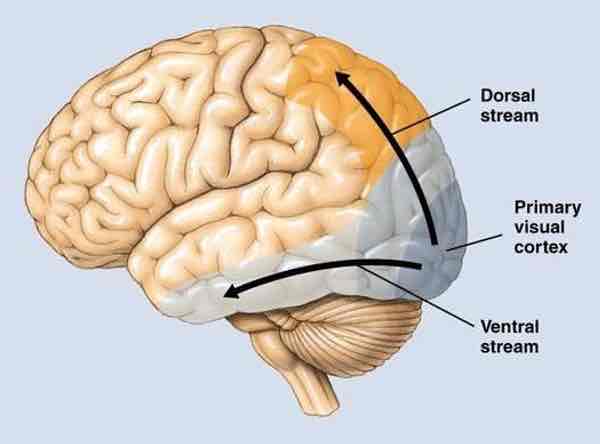
Dorsal pathway (extrastriate visual cortex)
”where/how“ pathway
Allows for locating objects in space, perceiving motion relative to other objects, making actions in relation to object
M & K pathway inputs
Vision for action pathway (1.Cells in V5 direction-selective 2.Cases of V5 parietal cortex damage→akinetopsia, apraxias)
From V1, info sent to parts of posterior parietal lobes & dorsal temporal lobes
Different regions specialized for identifying low-detail, movement-related visual info
Visual agnosia
Difficulty recognizing/interpreting visual information despite perfect vision
Often caused by damage to brain's visual processing areas
Various types affecting different aspects of visual perception
Object agnosia: difficulty recognizing objects
Prosopagnosia: difficulty recognizing faces
Spatial agnosia: difficulty perceiving spatial awareness
IT cortex
Face recognition region
Fusiform face area (FFA) in inferior temporal lobe activated in primates when viewing faces
Reduced FFA activity associated with poor face recognition (propropagnosia)
Akinetopsia
Motion blindness (stroboscopic vision→only snapshots of visual scenes, no continuous movement)
Caused by damage to V5 area (medial superior temporal lobe)
Ablation
Destruction of tissue
Electrolytic: electrode used to burn small area of tissue
Non-selective: all tissue at site destroyed
Exitotoxic ablation/lesions
Injection of excitatory agonist drugs directly into brain tissue
Selective way → only dentrites, cell bodies that have receptors for drug are affected
Temporary ablation/lesions
Injection of inhibitory agonists like muscimol (gaba-a agonist)
Way of inhibiting regional activity, drug effect washes out with time
Light microscopy
Resolution up to 1500x
Electron microscopy
Resolution up to the nanoscale (x10-9)
Synapses & individual organelles visible
Scanning electron microscopy
Resolution up to the nanoscale (x10-9)
Gives 3D view
Tracers
Used to identify projections by injection
Anterograde: dendrites→axons (eg. Phaseolus vulgaris leucoagglutinin)
Retrograde: axons→dendrites (eg. Fluoro-gold/ hydroxystilbamidine)
Computerized axial tomography (CAT or CT)
Pass x-rays through head
Shows tissue density
Magnetic resonance imaging (MRI)
Uses magnets & radio waves to determine tissue density in brain
Diffusion tensor imaging (DTI)
Form of MRI
Tracks water movement along myelinated axons to show where white matter bundles are
Positron emission tomography (PET)
Radioactive glucose injected into bloodstream
Brain tissue that is active uses more glucose
Functional MRI (fMRI)
Detects small changes in oxygen use in active brain areas
No radioactivity needed
Regions of tissue that are active require more oxygen & more oxygenated blood flows to active brain regions
Measures blood oxygenation level-dependent (BOLD) levels
Microdialysis
Measures release of neurotransmitters within brain region
Dialysis probe inserted into a brain region
Substances in extracellular fluid can diffuse across membrane into tube
Analyze fluid sample for content
Electrophysiological recordings
Listen in on cell firing patterns from single cell all the way up to multiple brain regions at once
Use electrodes to directly stimulate cells/specific parts of brain
Lateral hypothalamic stimulation: animals will self-administer stimulation to electrodes near lateral hypothalamus (activate dopamine cells in ventral tegmental area)
Transcranial magnetic stimulation (TMS)
Generates mild magnetic fields to trigger changes in activity of localized brain regions
Used for research & therapeutic purposes
Allows for non-invasive manipulation of neural activity
Device held near skull surface, pulsed current through device creates magnetic field which alters neural activity in tissue below the skull
Vs TDCS → pulsed current directly through
Deep brain stimulation (DBS)
Brain stimulation techniques increasingly used for therapeutic purposes in humans
Implantation of chronic electrodes for some movement disorders (Parkinson's), severe depression, epilepsy
Optogenetics
Activate parts of brain without electricity or drugs
Provides selective way of activating/inhibiting specific neurons by “injecting“ light into brain
Infect cells with viruses so they make “light-sensitive” receptor protein
Channel Rhodopsin 2→activated by blue light→ opens Na+ channel
Halorhodopsin→activated by yellow/orange light → open Cl- channel
Genetic methods
Manipulating gene expression allows targeting of specific proteins in nervous system
Multiple approaches (genetic mutation→delete gene, add extra copies, modify it; RNA manipulations→block production of proteins from gene transcription)
Manipulation/mutation: targeted deletion of a gene (‘knockout‘) can tell us about role a gene may normally have
Crispr-CAS9, knockouts/knock-ins, upregulation|downregulation, cross-breeding
Experimental controls
An equivalent experience of all steps of a manipulation/treatment except for step that is thought to have an effect
For surgery→’ sham’ surgery
For drug injections→vehicle injection (eg. Saline)
Recognize different ways of lesioning brain tissue
Electrolytic
Excitotoxic
Temporary
Recognize different ways of imaging/recording activity of live brain tissue
Microscopy: light microscopy, electron microscopy, scanning electron microscopy
Tracers: anterograde, retrograde
Brain imaging: CAT, CT, MRI, fMRI, DTI, PET
Recognize different ways of manipulating of live brain tissue
Lateral hypothalamic stimulation
Transcranial magnetic stimulation
Transcranial direct current stimulation
Deep brain stimulation
Optogenetics
Genetic manipulation/mutation
Physical properties of sound
Produced by objects that vibrate & set molecules of air into motion
Vibrations cause molecules of air to move in waves that travel away from object
Frequency, measured in hertz (Hz), cycles/sound
Hz determines pitch of sound (high→soprano, low→bases)
Loudness/volume reflects how ‘big‘ sound waves were
Timbre is complexity of sound waves
Anatomy of auditory system
Outer
Middle
Inner
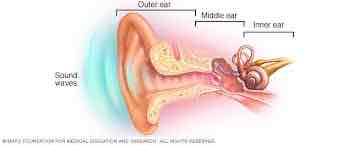
Outer ear
Pinna funnels sound waves into auditory canal
Tympanic membrane (eardrum) at end of canal
Middle ear
Tympanic membrane→ossicles: hammer (malleus), anvil (incus), & stirrup (stapes) bones
Stirrup pushes on oval window membrane
Inner ear
Oval window→opening into cochlea
Fluid filled→ fluid movement creates travelling wave in membranes inside cochlea
Specialized membranes inside
Organ of Corti
Region where sound wave transduction happens
Basilar membrane (bottom)
Tectorial membrane (tap)
Hair cells (outer & inner)→attached to basilar membrane, some touch the tectorial membrane above
Hair cells (auditory system)
Sound receptors
Inner & outer rows of hair cells on basilar membrane
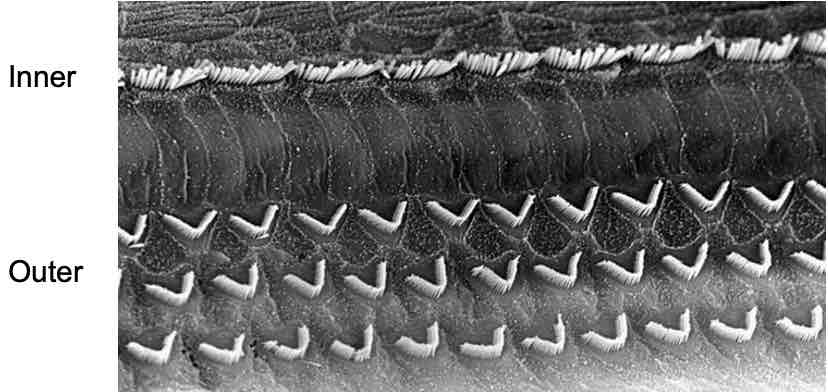
Sound transduction
Each hair cell contains cilia
Side-by-side, attached to one another by flexible tips links (movement of one cilium pushes/pulls the ones around us)
Site where tip links attach to each cilium called insertional plaque
Insertional plaques contain ion channels
Movement of cilia opens ion channels in insertionnal plaques
Ion channels open→ ions flow→electrical changes inside hair cell (receptor potential)
Pitch
2 mechanisms used in cochlea: Place coding & Rate coding
Place coding: high frequency (high pitches) move only part of basilar membrane closer to oval window
Rate coding: hair cells on basilar membrane match firing rate to frequency of sound ( 100 Hz → hair cells fire 100 times/second), rate of cell firing in auditory nerve gives us info about sound pitch
Evidence shows that both place & rate coding happen in cochlea (place coding→higher pitches, rate coding→ lower pitches)
Auditory pathway in brain
Auditory nerve activated
To medulla (cochlear nucleus)
To tectum in midbrain (inferior colliculus)
To thalamus (medial geniculate nucleus)
To primary auditory cortex (temporal lobe)
Each hemisphere ‘hears‘ from both ears, but ‘hears more‘ from contralateral ear (left ear→right temporal lobe)
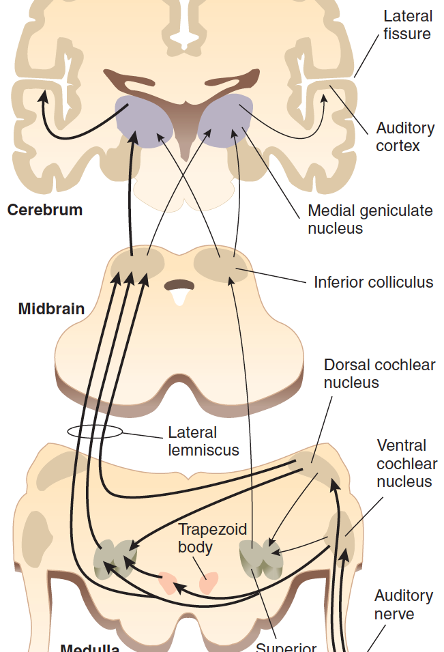
Causes of deafness
Conduction deafness: ears do not conduct sound to nervous system
Sensorineural deafness: problems with structures that convert sound vibrations into neural activity
Central deafness: hearing loss caused by brain damage (variable forms)
Causes of deafness in aging:
Presbycusis: most common form of a hearing loss in older adults, hearing loss greater for high pitched sounds
Hair cell loss: noise, genetic variation, some medications (aspirin & certain antibiotics)
Loss of blood supply to ear: heart disease, high blood pressure, diabetes
Damage to middle ear (membrane or bones conducting sounds)
Treating deafness
Cochlear implants used to treat deafness due to hair cell loss (sensorineural or central deafness)
Electrical currents stimulate the auditory nerve
Complex sound processing
Hearing in cerebral cortex occurs through 2 pathways (anterior & posterior)
Anterior pathway: ‘what‘ pathway, helps us recognize what sounds are, judge if familiar/ unfamiliar
Posterior pathway: ‘where‘ pathway, helps us identify where sounds are coming from
Evidence shows that these system are plastic
Music/pitch perception
Musicians have larger neural response to pitch info than non-musicians (shown for both musical & speech sounds)
Musician’s brains look different & respond differently to music (activation of motor regions when listening to familiar piece)
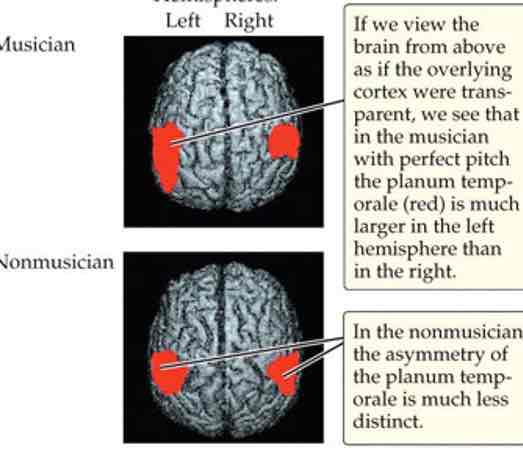
Taste: physical stimulus
Taste involves perception of chemical compounds (tastants) dissolved in medium (not same as flavour)
Humans perceive only a limited range of tastants (each may have own adaptive/survival value, responses to each can change with age/experience/cultural influences)
Taste (anatomy)
Tastant receptors found in taste buds on tongue, mouth & throat (on tongue, taste buds located on sides of papillae)
No tastant-specific ‘map‘ of tongue
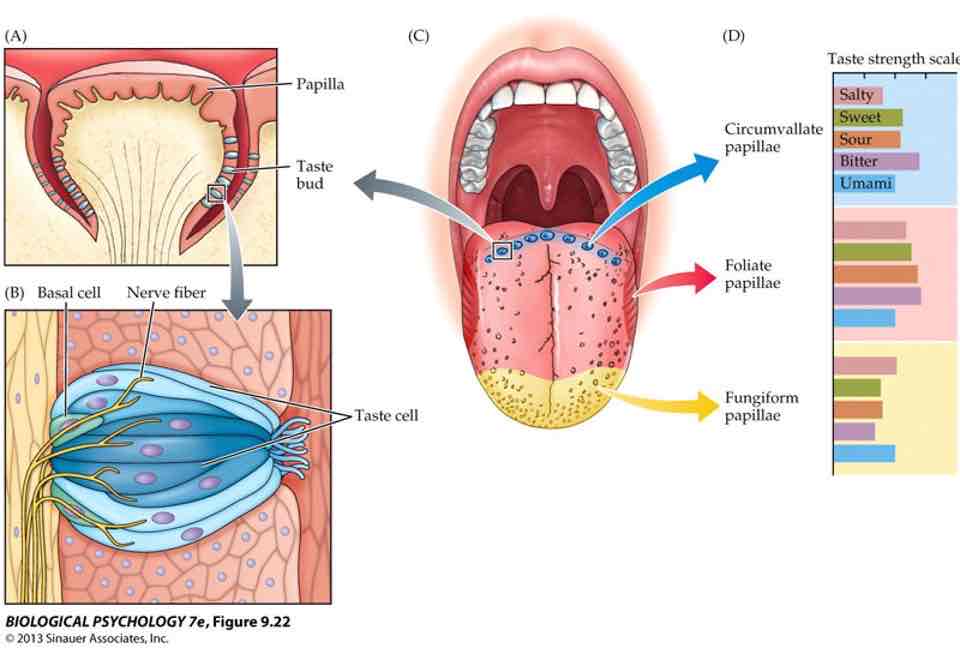
Taste: transduction
A taste bud is a cluster of receptor cells (constantly regenerating)
Each cell extends microvilli into ‘taste pore‘ (gap between papillae)
Microvilli contain ion channels/receptors
Tastants bind to receptors→triggering transduction
Change in electrical state of taste cells, synapse with sensory nerve fibers in tongue
Each taste cell responds to only one type of tastant
Taste: trajectory
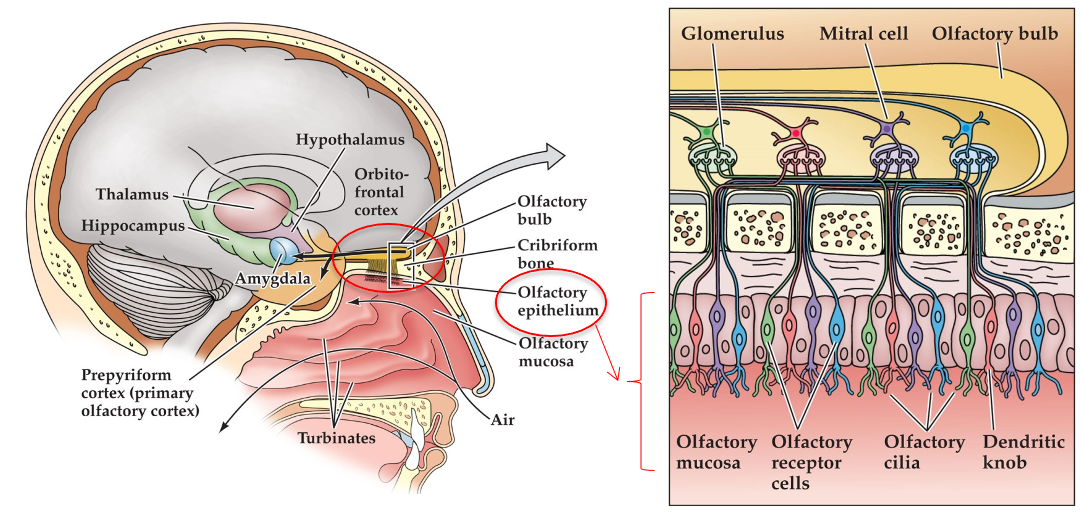
Taste signals carried to brain via cranial nerves
Brainstem: nucleus of solitary tract
Ventral posteriomedial thalamus→primary gustatory cortex= frontal cortex (insula) →orbitofrontal cortex
Ventral posteriomedial thalamus→somatosensory cortex
What affects the sensitivity of our sense of taste?
Absolute thresholds very low for bitter tastants
Higher for sweet tastants
Combinations can modify perceived intensity of each
Interactions with other senses (smell)
A factory triggered by airborne molecules: may be inhaled directly from outside air or pushed up through back of mouth/throat into naval cavity
Smell: anatomy
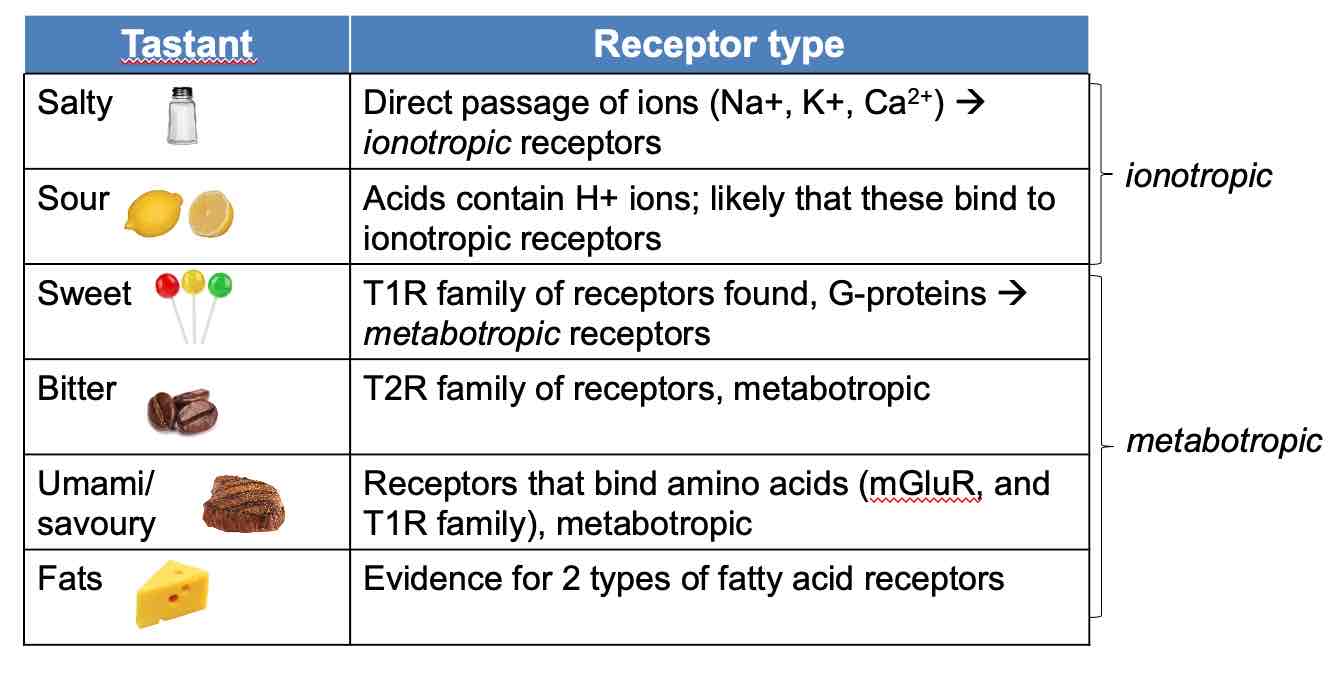
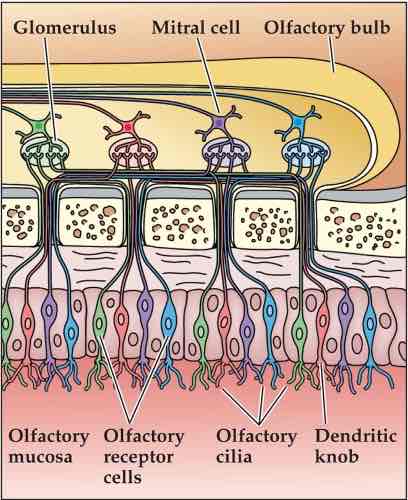
OIfactory receptor cells are bipolar cells
Axons project up through cribiform bone ( axons terminate in glomerulus, small clustered region of axons)
Mitral cells in olfactory bulb, brain tissue above cribiform bone (mitral cells send their dendrites into glomerulus, axons of mitral cells form olfactory tract into brain)
Olfactory epithelium
Olfactory epithelium
Layer of tissue at top of nasal cavity
Receptor cells embeddedin epithelium
Cilia project into mucosal layer
Smell: transduction
We can distinguish between 1000s of different smells
In humans,~350 different olfactory receptors made (all metabotropic)
Each receptor can bind to more than one type of odorant molecule
Each odorant can bind to more than one type of receptor
Individual smells created by unique patterns of receptor activation
What affects our sensitivity in our sense of smell?
Type of odorant (different absolute thresholds for different odorants)
Developmental differences (hyposmia-decreased ability to smell-more common in older age)
Sex differences (women > men, small differences for some odorants)
Experience ( adaptation, rapid sensitivity decreases with prolonged exposure to odorants)
Influenced by attention (less adaptation to noxious smells)
Recognition thresholds influenced by familiarity/experience (also affected by verbal abilities, greater vocabulary = greater recognition)
Multimodal nature of flavours
Vision: visual can affect how flavour is perceived (eg. White wine with tasteless/odourless red dye described by oenologists as red wine), reported sweetness enhanced by red; saltiness by white, congruence between visual cues & smells, flavours affect judgements of un/pleasantness
Hearing: ’sonic chip’ experiment (higher crispness, freshness ratings with louder, high-frequency sounds), high-frequency sounds increase ’fizziness’ ratings of carbonated drinks, high levels of background noise may reduce perceived saltiness
Hedonic value of flavours (like/dislike)
Orbital frontal cortex is key
Activity in OFC correlates with motivation to consume, ratings of pleasantness of foods
Activity in OFC correlates with expectation of quality & with rated ‘liking‘ of wine & with believed price
Vestibular system
Allows perception of ‘linear translational movements’ in space & rotational movements of head
Sense of balance
Keep head in an upright position
Adjusts eye movement to compensate for head movements
2 parts: semicircular canals & vestibular sacs
Semicircular canals
Orientation of head space
3 fluid-filled canals
Movement of head causes movement of endolymph
Ampulla at base of each canal
Cupula within each ampulla contains hair cells
Movement of cupula causes movement of hair cells (bending of hair cells causes receptor potential, receptor potential activates auditory cranial nerve
Vestibular sacs
Utricle & saccule → balance & equilibrium
2 fluid-filled compartments next to semicircular canals (tissues on sides of sacs contain layer of hair cell receptors - otoconia on top of this layer)
Movement of fluid within sacs pushes on ocotonia, pushes hair cell cilia
Receptor potential activates auditory cranial nerves
Somatic perception
Different aspects to somatic perception (perception of the body) → 1. Cutaneous perception 2. Proprioception & kinesthesia
3 types of info received through cutaneous perception: touch, temperature, pain
Touch
Different types of touch (tactile) receptors give us different types of touch sensations → pressure, vibration, stretching, movement, textures
Tactile receptor types differ in:
What type of stimulation they respond to best
How fast they adapt (stop responding to stimulation)
Which type of skin they are found in (hairy vs glabrous) & where
Receptive field size
Cutaneous perception
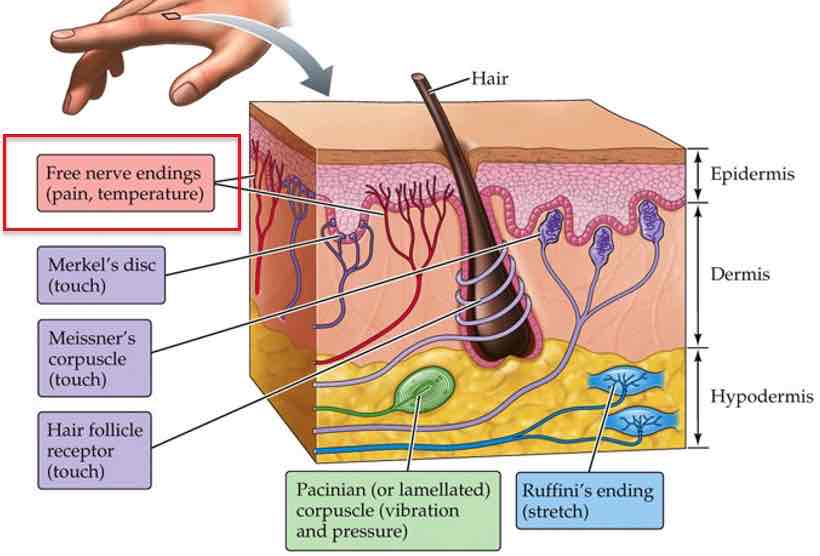
4 key types of ‘encapsulated mechanoreceptors‘, or tactile receptors
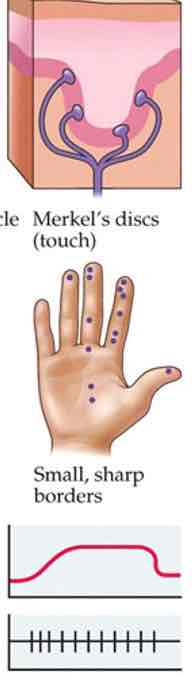
Merkel's discs
Meissner's corpuscle
Ruffini's ending
Pacinian corpuscle
Merkel's discs
Touch
Fingertips, lips, tongues
Pressure info
Form perception, edges, fine details
Slow adapting
Small receptive fields
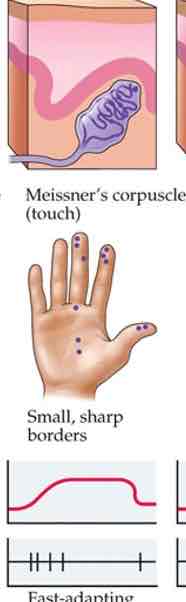
Meissner's corpuscle
Fingertips, lips, tongue
Pressure & movement info
Form perception, texture
Fast adapting
Small receptive fields
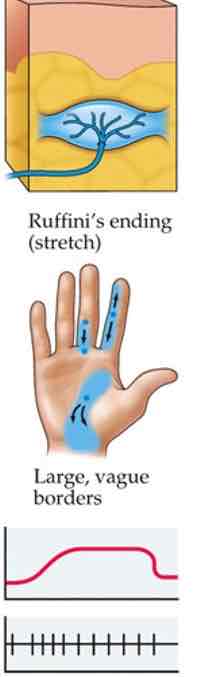
Ruffini's ending
Deeper, in hypodermis
Stronger pressure
Stretch info
Slow adapting
Large receptive fields
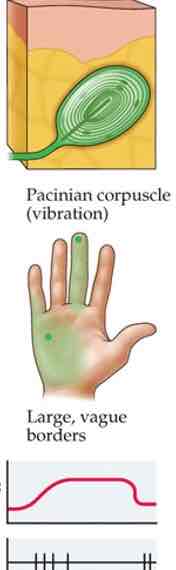
Pacinian corpuscle
Deeper, in hypodermis
Strong, vibrating pressure or movement
Texture info
Large receptive fields
Temperature perception
Temperature receptors in skin respond to changes in skin temperature from 34°C (normal)
’Transient receptor potential‘ (TPR) receptor family
Transient receptor potential (TPR)
Cold sensors in skin closer to epidermis, warmth sensors located more
Warmth sensors located more deeply
Each TPR receptor type has a ’preferred‘ range of temperature
Some can be activated by chemical chemical compounds
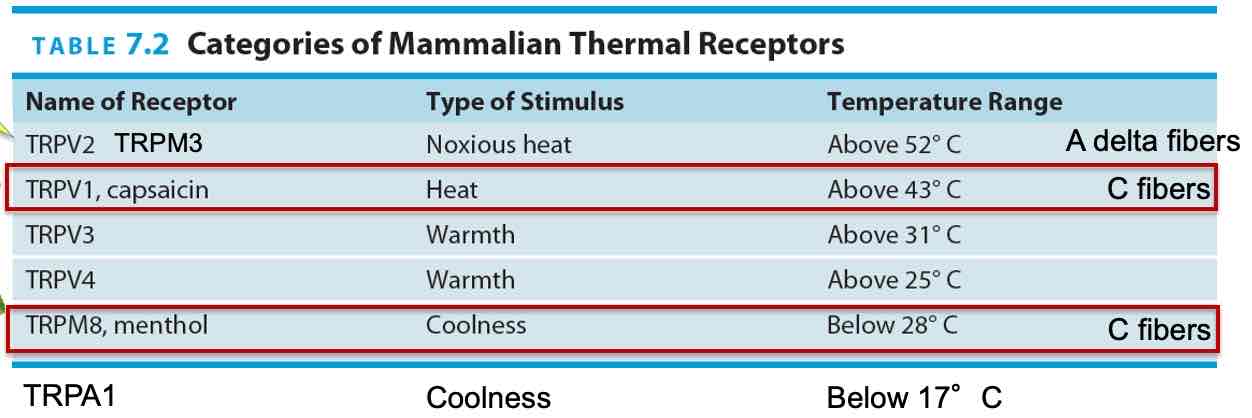
Nociception
Pain perception = ‘nociception‘, noxious or harmful
Several types of pain receptors in skin on free nerve endings
TRPV1, TRPV2 receptors activated by strong heat chemical compounds
At least 4 types of pain receptors in skin
High-threshold mechanoreceptors
TRPV1 receptors: heat (burns) & chemicals (eg. Capsaicin), burning sensation
TRPA1 receptors: chemicals that cause inflammation
TRPM3 receptors: activated by very high h eat ( around > 45°C → higher TRPV1), axons of nerves large diameter (myelinated)
Activation of receptors→A delta or C fibers in spinothalamic path (special kind of sodium channel found in these axons: Nax 1.7)
Cingulate cortex activity correlates with amount of pain reported
Cingulate activity decreases when painkillers (analgesics) or placebos taken & reported pain decreases
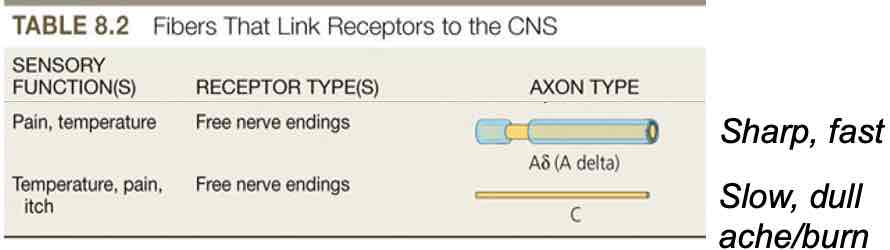
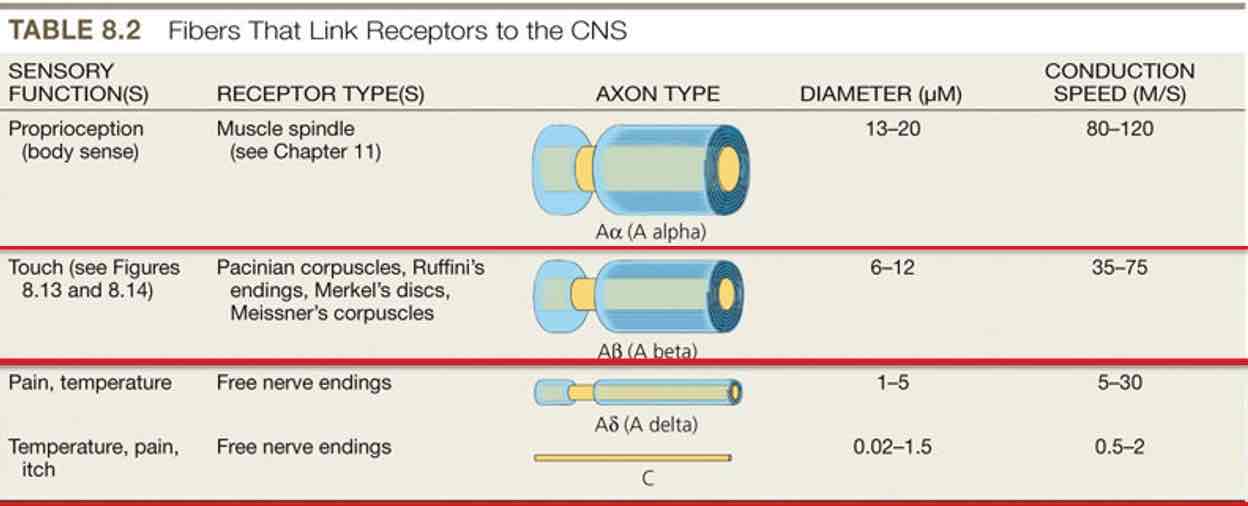
Somatosensory pathways
Somatosensory receptors (touch, temperature, pain) activated
From receptors in body below head, axons enter CNS via spinal nerves (dorsal root ganglia)
From face & head, via cranial nerves (mostly trigeminal nerve)
Route in CNS depends on type of info carried: dorsal column (touch) & spinothalamic (pain, temperature)
Both pathways send axons to: ventral posterior thalamus→primary somatosensory cortex
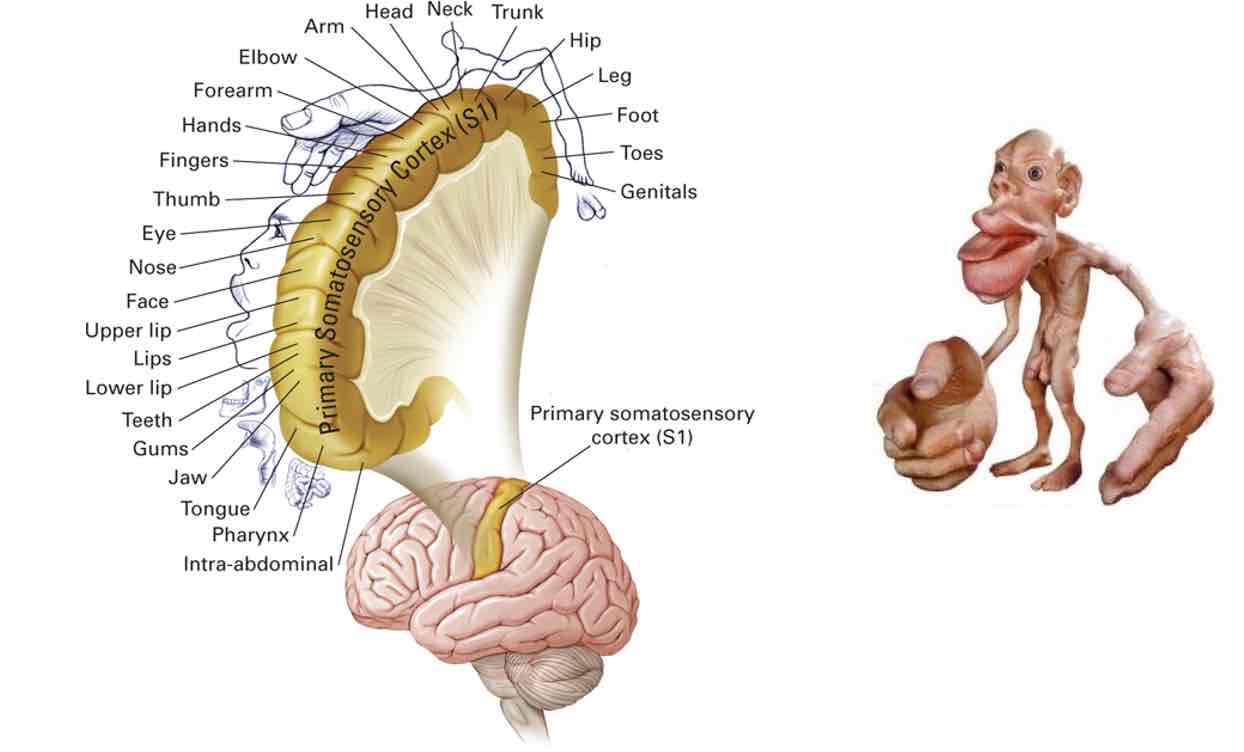
Primary somatosensory cortex
Somatic map of body in parietal cortex
Bigger parts → more sensitive to touch/temperature /pain perception
Somatic nerves
Tactile information is carried by different types of nerves than temperature or pain info
What happens if transduction pathway mutated (Nax 1.7)
Ashlyn Blocker → “ pain is a gift, she doesn't have it”
Inherited erythromelalgia “man on fire”syndrome →hyperactivation of nerves carrying pain signals to spinal cord
Pain perception
Pain information also activates other regions of cerebral cortex: anterior cingulate cortex (ACC) & insular cortex (frontal lobe)
Different parts to experience pain:
Touch, sensory → primary somatosensory cortex
Emotional response → ACC & insular cortex
Touch & pain interaction
Touch, pressure can sometimes suppress pain, at least temporarily
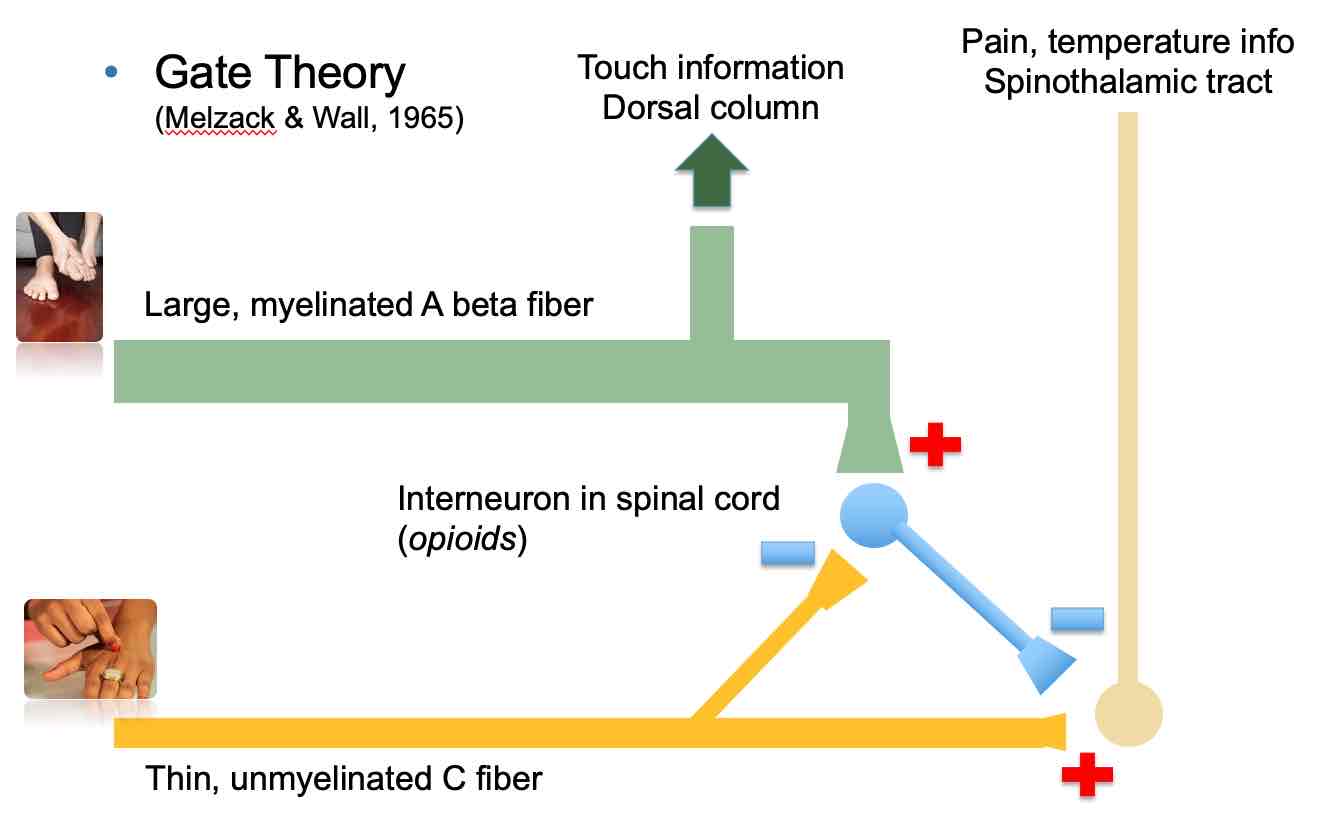
“Gate Theory“ of pain perception
Gate Theory