Lectures 8, 9 + flipped: Synapses and Neurotransmitters
1/96
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
97 Terms
Define synapse
Junction between 2 neurons allowing signals to pass from one to the other.
Define synaptic transmission
Process of signalling via synapses.
Why is it important that synapses enable flexible processing?
Might not always want muscle to move. Can adapt to diff cues coming in + integrate info.
Define interneuron
Type of neurons located between sensory + motor neurons, Make up majority of neurons in the body. Play a role in integrating sensory info + regulating motor activity.
How do you tell if neurons are connected by gap junctions?
Knock out connexin gene (gene usually allows hyperpolarisng + depolarising stimuli to be passed from one neuron to another) = first neuron fires but second does not have depolarisation or hyperpolarisation.
What are the benefits of gap junctions/electrical synapses?
Fast communication which allows for synchronising neurons.
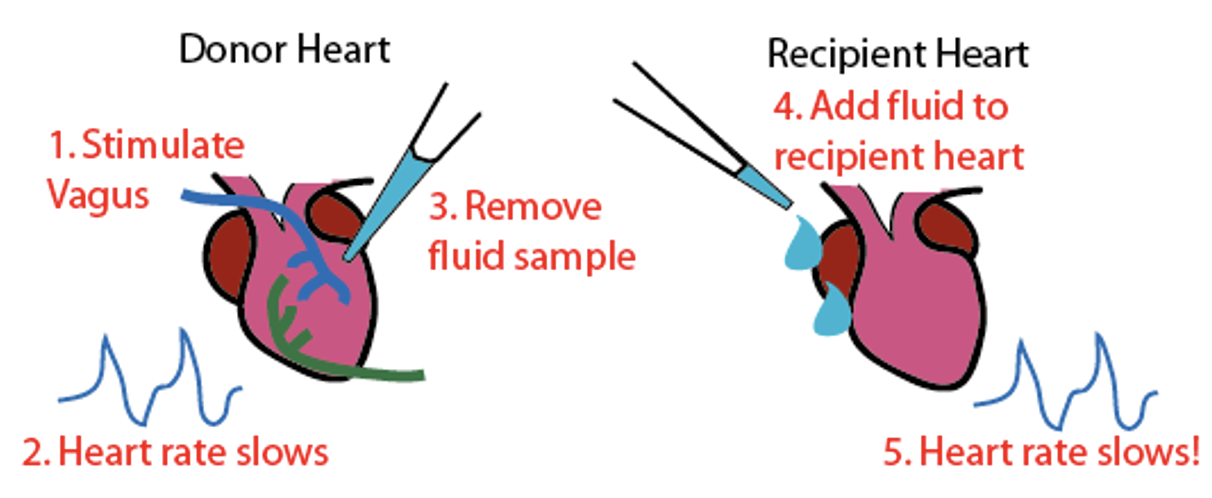
What was the first evidence of chemical synapses?
Used 2 isolated frog hearts to show that nerves release a chemical that slows heat beat. Removed fluid from donor hear + added to recipient heart = HR slows = knows chemical caused HR to slow.
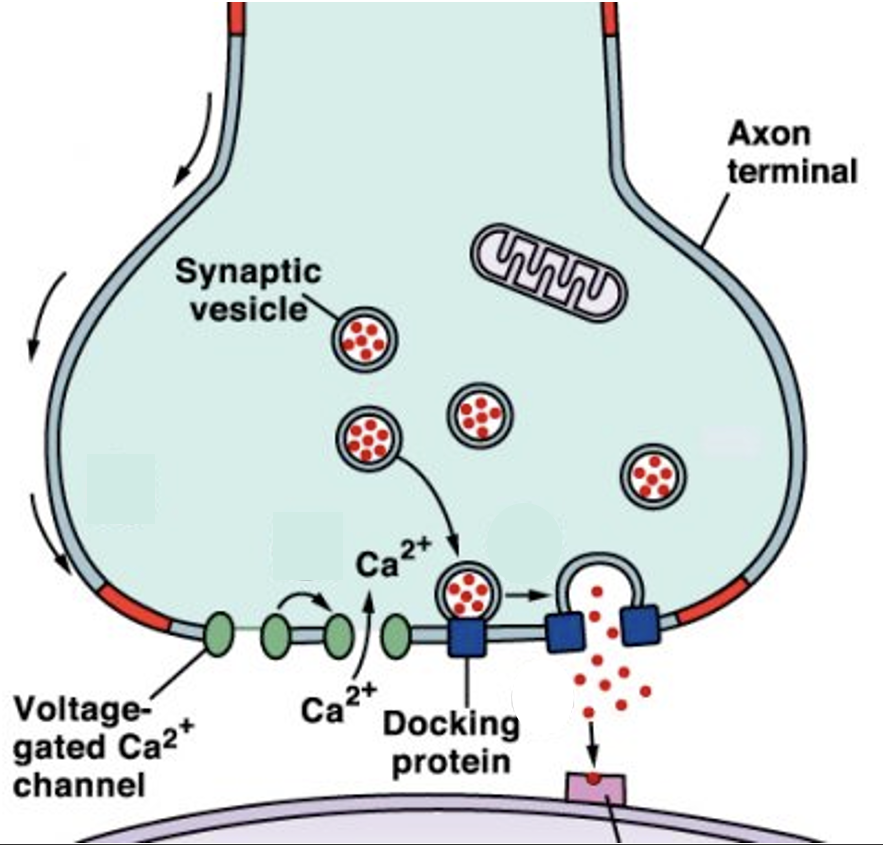
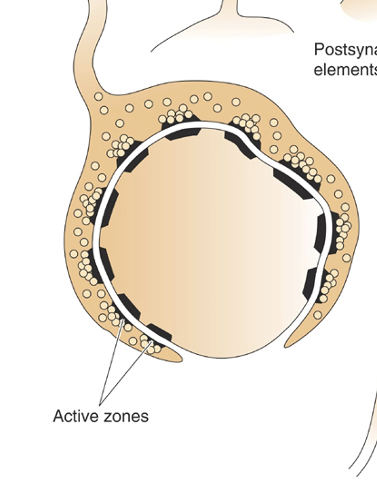
Define active zone
Where NTs are released. Lots of synaptic vesicles present.
Outline the steps in chemical synaptic transmission
Package NTs in vesicles, put them at pre-synaptic terminal.
AP arrives → VG Ca2+ channels open.
Ca2+ influx → vesicles fuse to men, NTs released.
NTs diffuse across the synaptic cleft, activate receptors on postsynaptic cell → further signalling.
NTs are removed from the cleft.
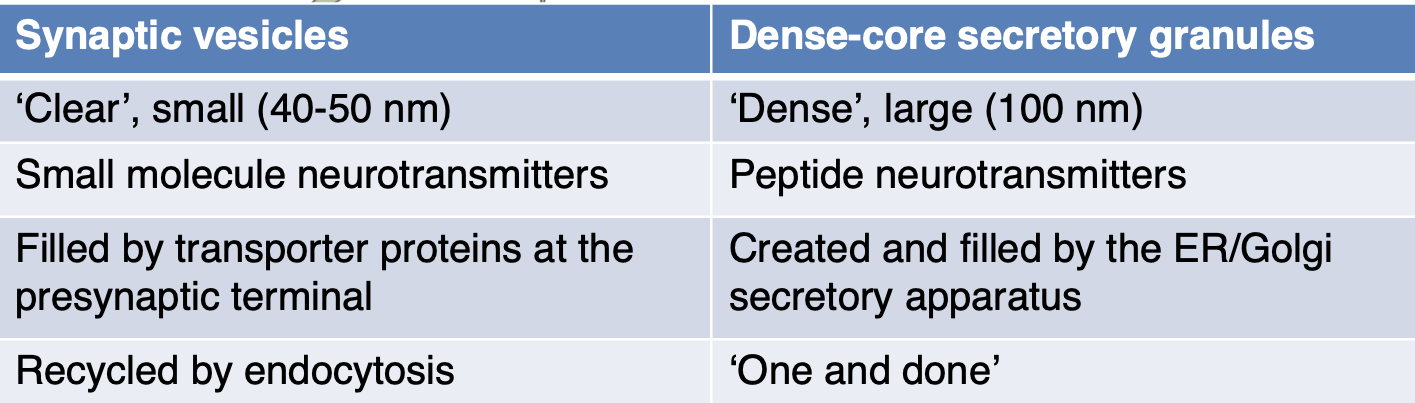
State the differences between synaptic vesicles + dense-core secretory granules
Conc Ca2+ outside cell = 1mM. Conc Ca2+ inside cell = 0.0001mM. When VG Ca2+ channels are opened, do you predict the net influx or outflow of Ca2+ ions + why?
Influx of Ca2+ down conc gradient, inside mem pot is more -ive, Ca2+ +ively charged, won’t stop flowing until mem pot becomes more +ive.
What does a high concentration of NTs allow for?
Higher chance than NTs will bind to receptors on the post synaptic neuron.
After fusing with the presynaptic membrane, by what process is the vesicle taken up again?
Endocytosis. (not for peptide NTs so this only happens for synaptic vesicles).
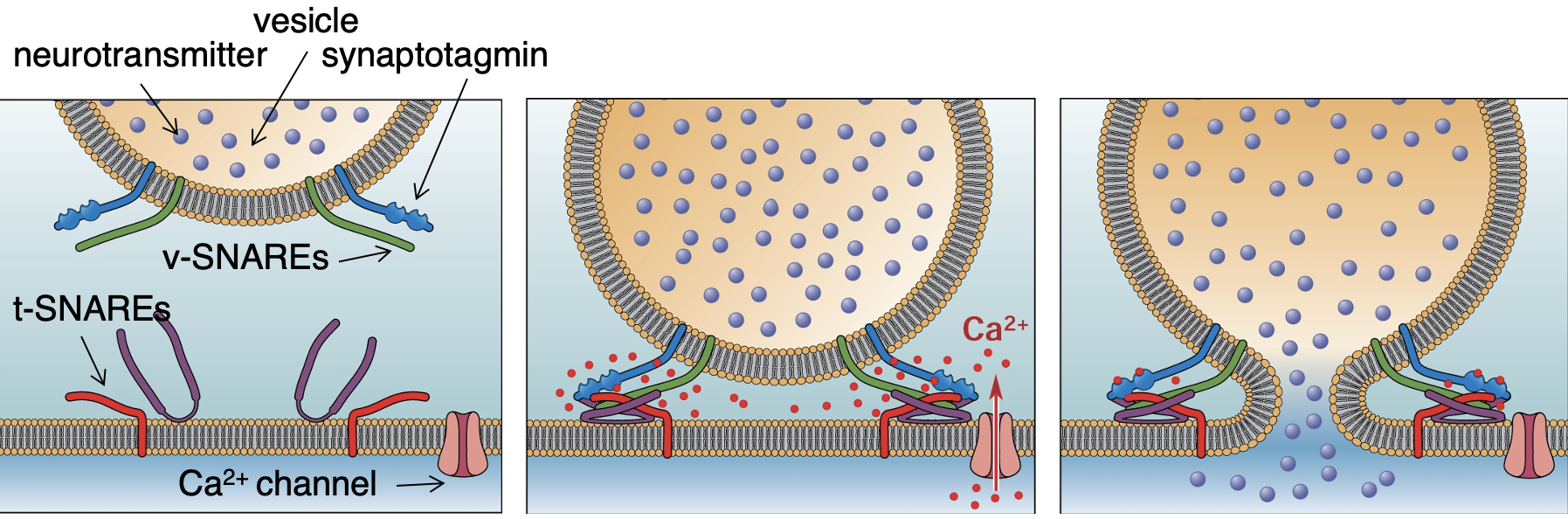
Define tSNARE, vSNARE + synaptotagmin
tSNARE: protein expressed on the membrane.
vSNARE: protein expressed on vesicle.
Synaptotagmin: protein that is able to bind to Ca2+.
Describe the process of how vesicles fuse via SNAREs
VG Ca2+ channels open = Ca2+ binds to synaptotagmin = conformational change in synaptotagmin = conformational change in tSNARE + vSNARE = 2 SNARES zipper together = forces vesicles towards membrane = force is high enough to cause it to fuse with the membrane = fusion allows for NTs to be released into synaptic cleft.
Why is botulinum toxin dangerous?
Cuts up SNARE proteins = no transmission as vesicles cannot be released = sometimes paralysis.
What other cellular processes aside from NT release require vesicle fusion?
Protein secretion, e,g insulin from pancreas cells.
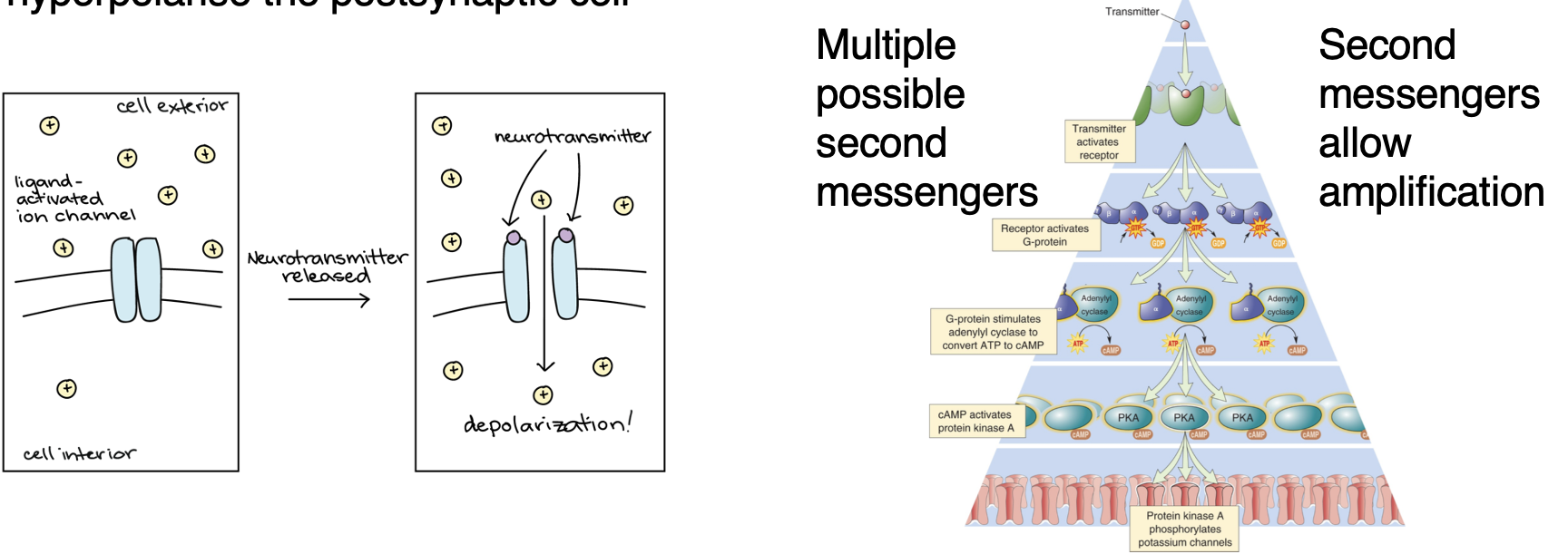
Describe how NTs affect the postsynaptic neuron by binding to receptors?
Ligand-gated ion channels (inotropic receptors) → directly depolarise or hyper polarise the post synaptic cell.
G-protein coupled receptors (metabotropic receptors) → more complex effects.
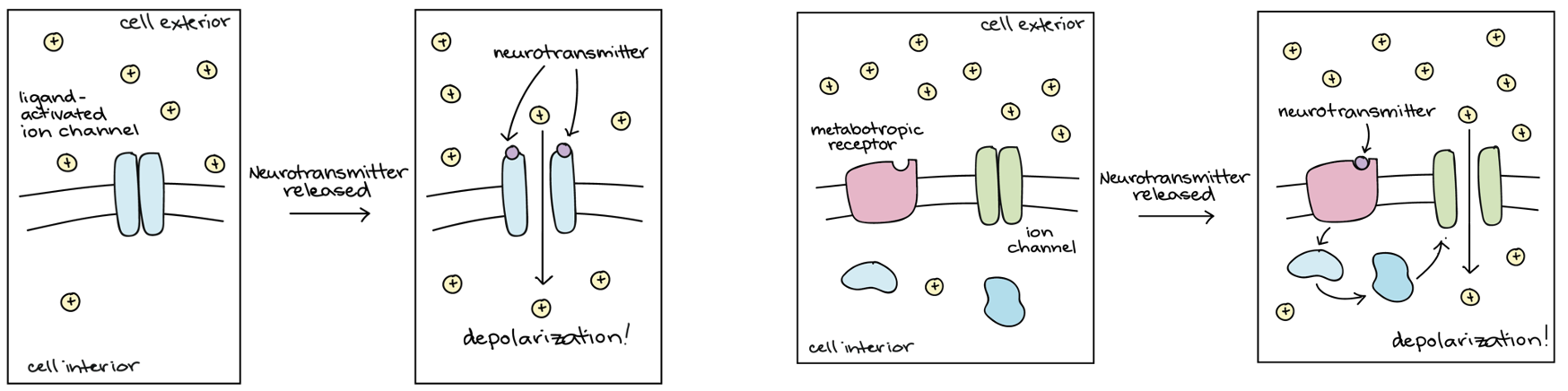
What are the 3 ways in which NTs are removed after synaptic transmission?
Diffuse away.
Actively taken up by transporters for recycling into the presynaptic neuron or glia.
Destroyed in the synaptic cleft by enzymes.
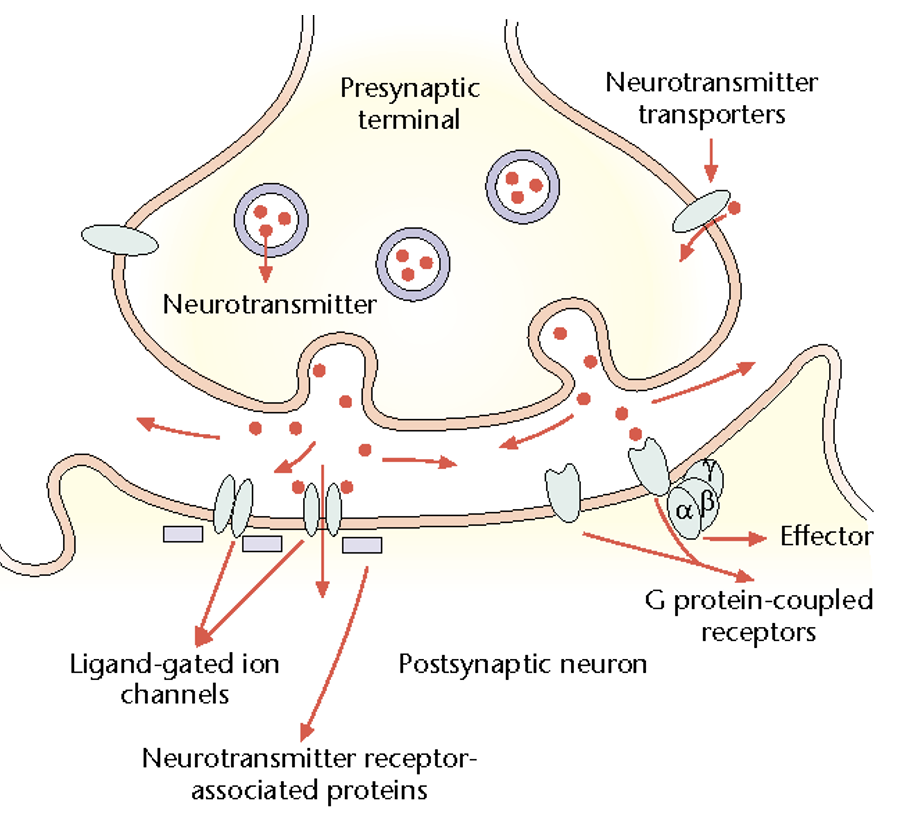
Describe differences + similarities between electrical + chemical synapses
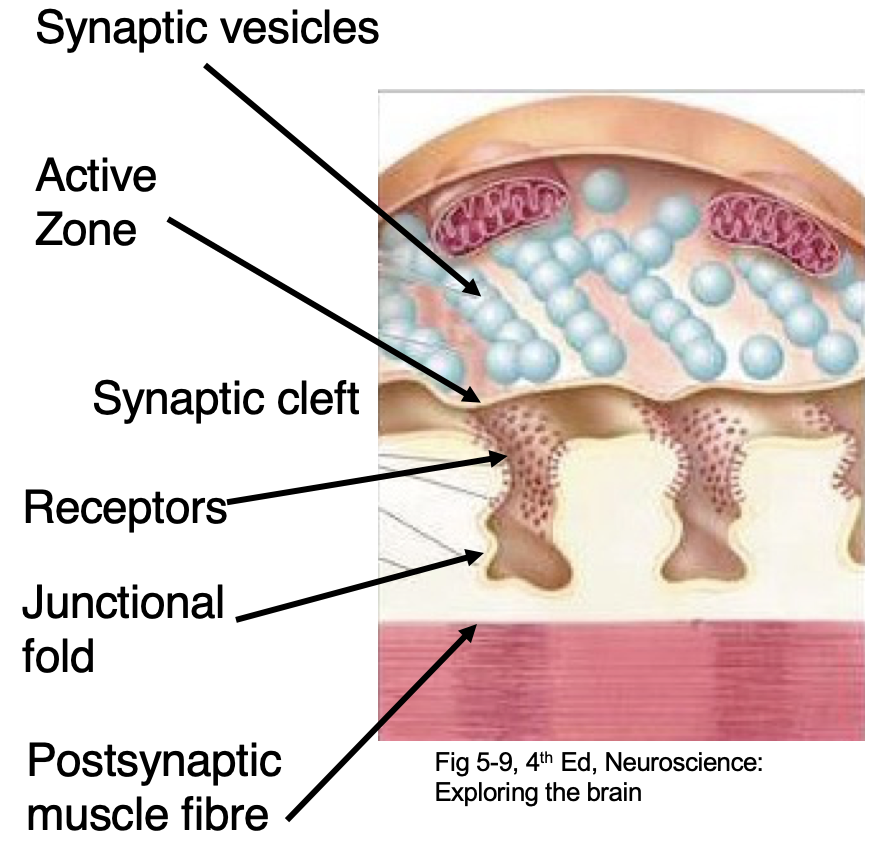
Define neuromuscular junction (NMJ)
Connection between motor neuron + muscle. Fast + reliable neurotransmission. Uses ACh.
How does the NMJ achieve efficient transmission?
Presynaptic neuron has lots of active zones. Postsynaptic muscle fibres has junctional folds (densely filled with NT receptors) which line up with active zones = higher chance of NTs binding + causing a change.
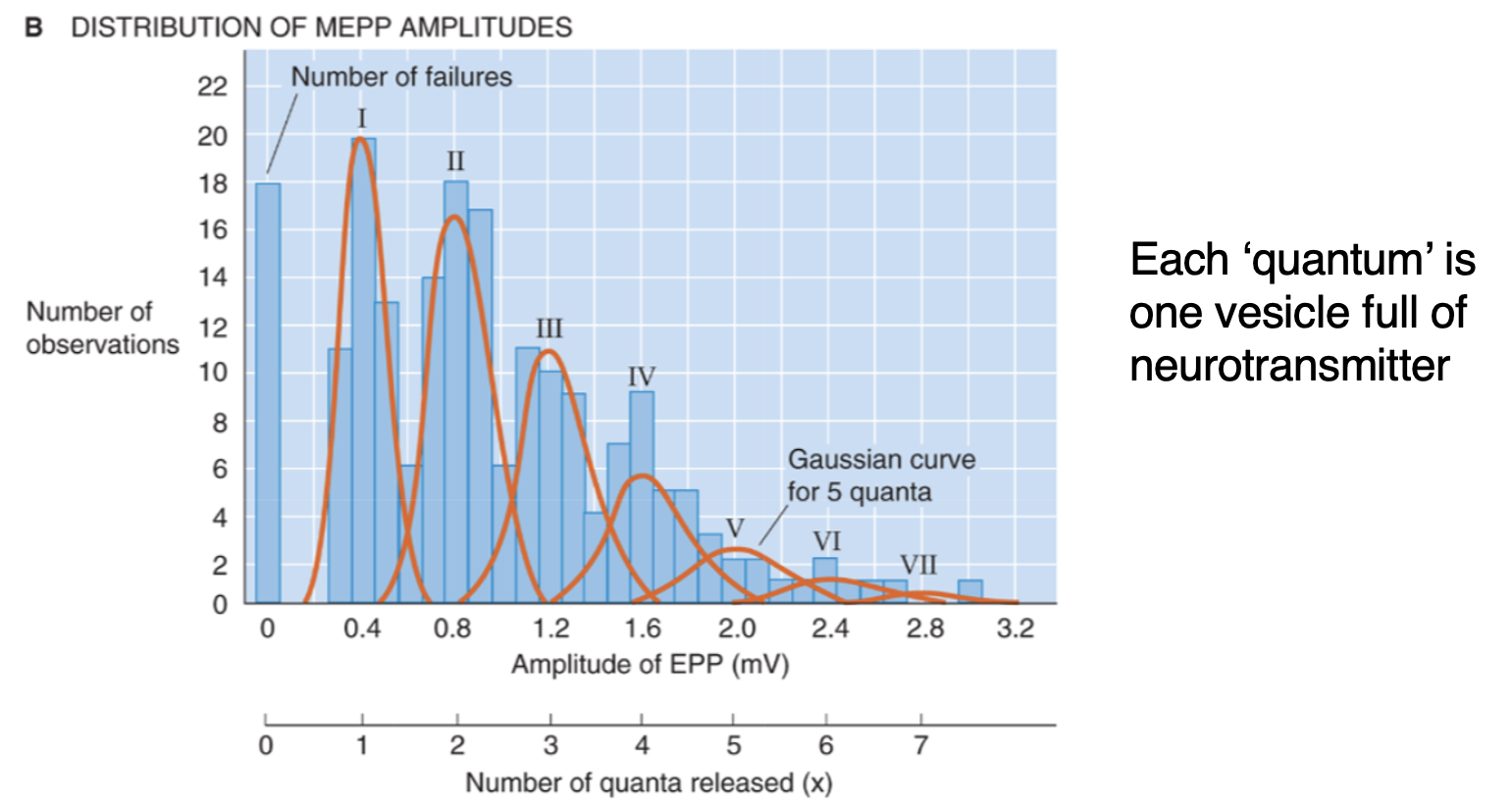
How was it discovered that NTs are released from vesicles?
When record from muscles with no stimulation = spontaneous MEPP (motor end plate potential) which are always the same size = must be repeatable way of releasing signal each time. When motor nerve is stimulated = jumps in amplitude potential are neatly aligned with integer multiples of the spontaneous MEPP. If NTs continuously released would expect to see continuous distribution which is not seen.
Give an example of how the shape of a synapse has adapted for its purpose
NMJ = one surrounds the other = active zones aligned = always want second neuron to fire following the first one.
State the criteria for NTs
Should be present in presynaptic terminals, released in response to stimulation, act on the postsynaptic neuron. Blocking the NT should prevent synaptic transmission.
What experimental technique can be done to determine is a NT is present?
Immunostaining.
What experimental techniques can be done to see if a cell expresses enzymes to synthesise NTs or if transporter proteins are used to store it?
Immunostaining, in situ hybridisation.
What can be done to see if a NT is released?
Collect fluid around neurons after stimulating them.
What can be done to see if a potential NT affects the postsynaptic cell?
Test if the molecule mimics the effect of stimulating the presynaptic cell.
How can a NT be blocked experimentally?
Apply drugs, delete genes encoding enzymes/transporters/receptors.
State 3 NTs that are a.a
Glutamate, GABA, glycine.
State 2 NTs that are amines
ACh, NE (norepinephrine).
State 2 NTs that are peptides
Opioids, endorphins.
State features of NTs that are a.a + amines vs peptides
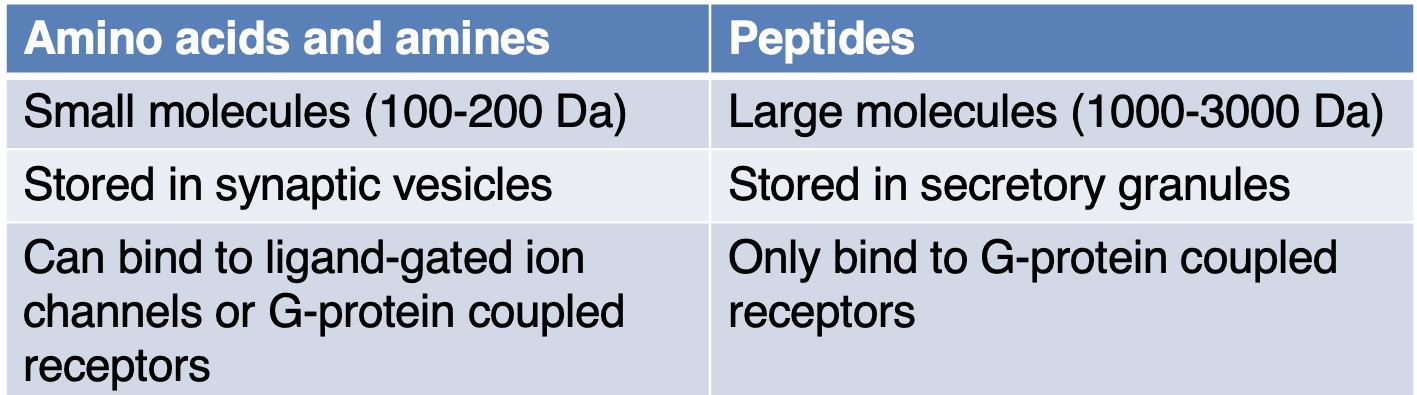
What other type of small molecule transmitter can peptide releasing neurons also release?
Co-transmitters.
Describe 2 types of NT receptors
Ligand-gated ion channels (inotropic receptors): directly depolarise or hyperpolarise the postsynaptic cell.
G-protein-coupled receptors (metabotropic receptors): more complex effects. Multiple possible second messengers which allow amplification.
Define divergence
NT activates one receptor but they often have diff subtypes which can the effect diff system = amplification of signal + wide range of effects due to one NT.
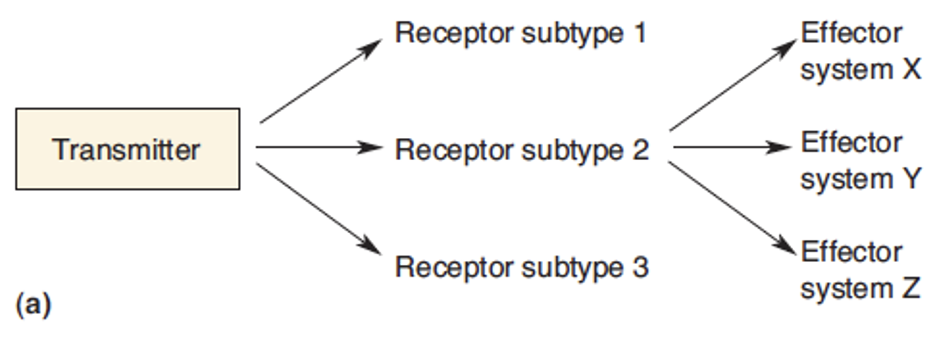
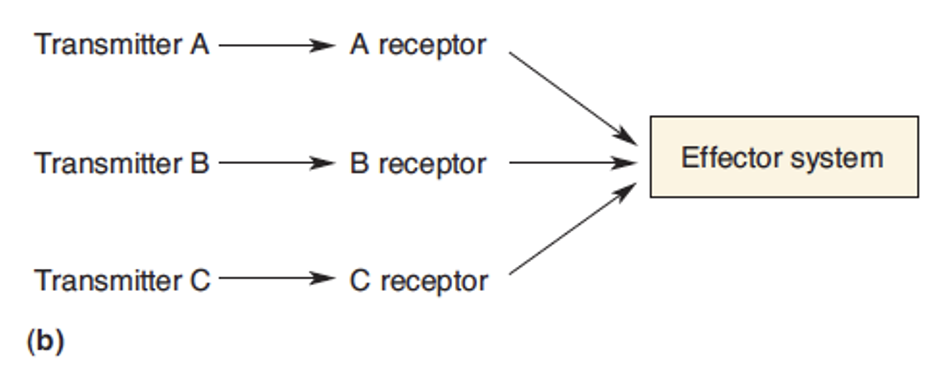
Define convergence
Diff receptors activate same system = increase chances of one system being activated.
Describe glutamate
Most common excitatory transmitters in CNS. an a.a = found in all neurons. 3 inotropic glutamate receptor subtypes based on the drugs which act as selective agonists. Action is terminated by selective uptake into presynaptic terminals + glia. Not released from all neurons, some use it in protein synthesis.
Describe AMPA receptors
Mediate fast excitatory transmission. Glutamate binding to AMPA receptors triggers Na+ + K+ currents = an EPSP. (*blue on diagram attached*)
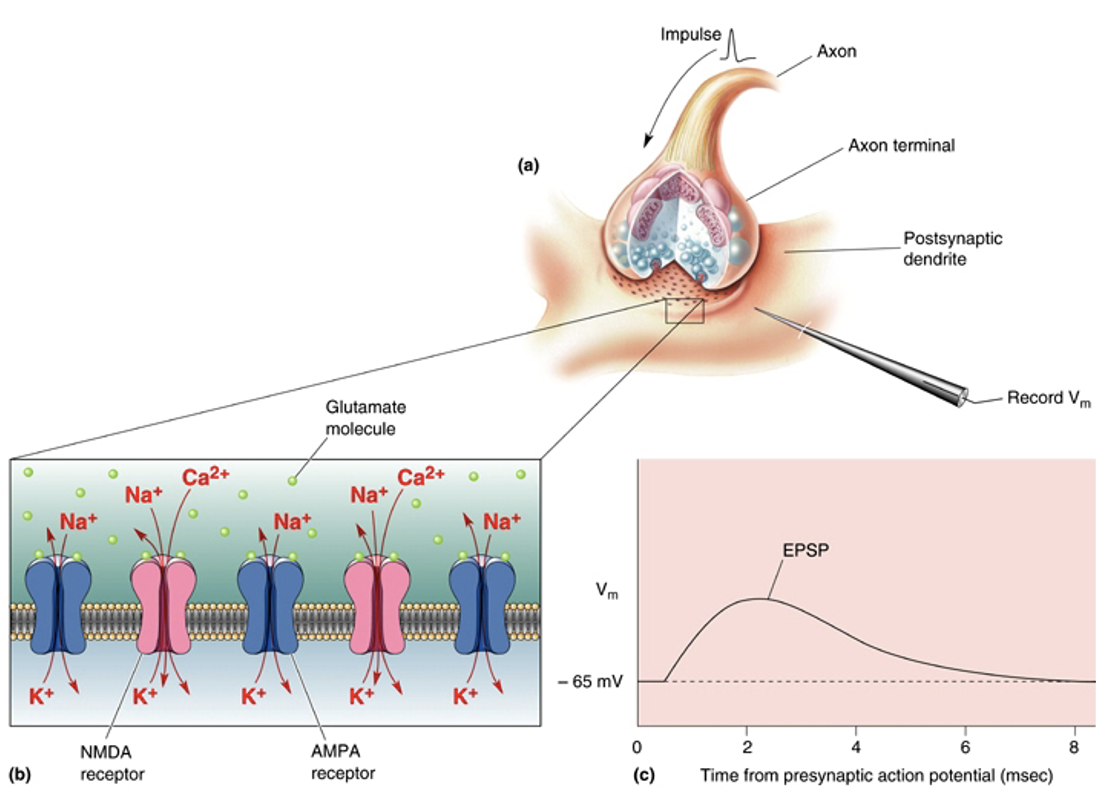
Describe NMDA receptors.
Often co-exist with AMPA. Have voltage dependent Mg2+ block = only opens when the neuron is already depolarised (then pushes Mg2+ ion out of pore = ions flow in + out). Let in Ca2+ = downstream signalling. Function as a coincidence detector (when a neuron is activated right after it was already activated).
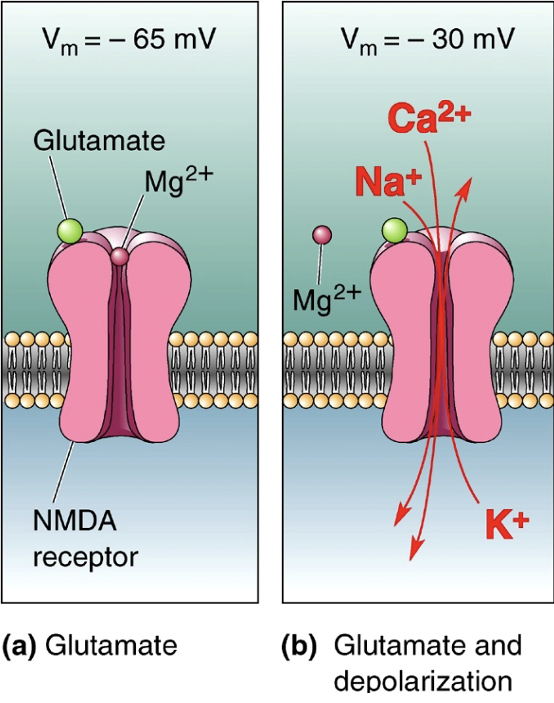
Why can G-protein coupled receptors (GPCRs/mGluRs) be beneficial?
Allow glutamate to be inhibitory = as not an ion channel = when NT binds to GPCR whatever other proteins it happens to activate could be excitatory or inhibitory depending on their function.
Describe GABA production
Not an a.a used to synthesise proteins. Synthesised from glutamate by the enzyme glutamic acid decarboxylase (GAD). Action is terminated by selective uptake into presynaptic terminals + glia.
What does GABA do?
Usually an inhibitory transmitter in CNS. Produces IPSPs via GABA-gated chloride channels (GABAa receptors), if the membrane potential is above chloride’s Nernst potential (slightly more +ive than the mems resting pot = at rest no inflow on Cl- in cell).
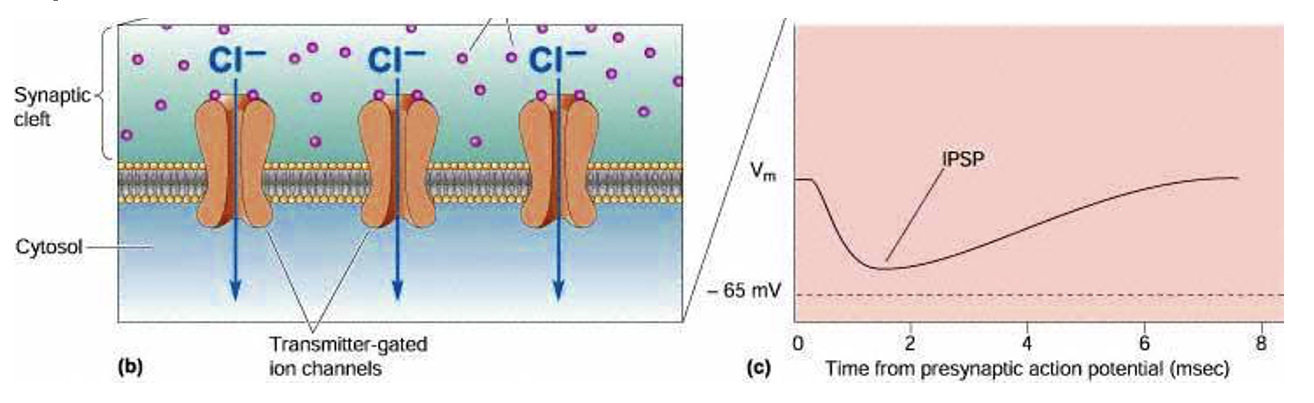
What happens if theres too little or too much GABA?
Too much = coma or loss of consciousness.
Too little = seizures.
When other chemical bind to GABAa receptors what also needs to be present?
GABA also needs to bind otherwise the drugs won’t have an effect (allosteric drugs).
What happens when ethanol binds to GABAa receptors?
GABA binds to channel + ethanol binds to other site on channel = enhances action of channel = more Cl- flow in = more hyperpolarisation = more inhibition.
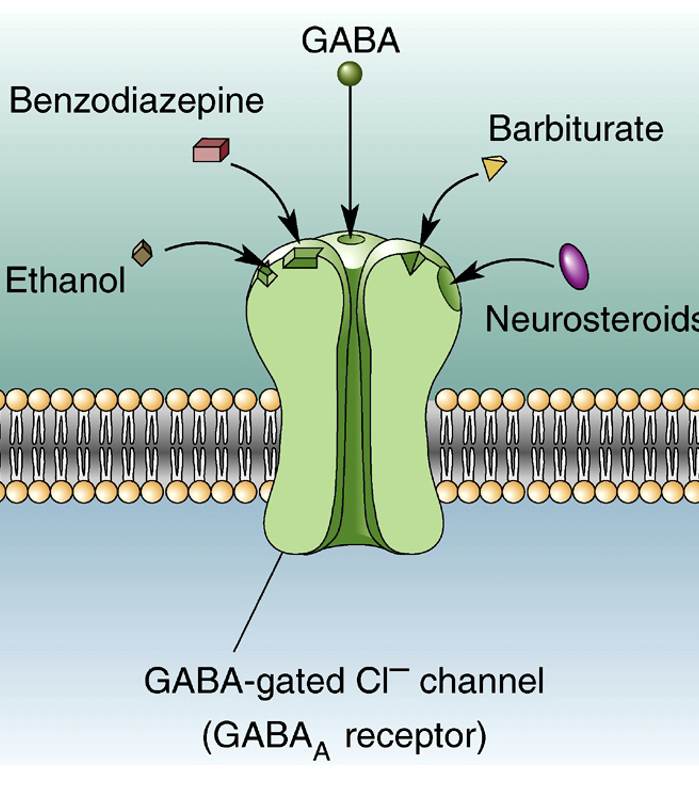
State 3 allosteric drugs that bind to GABAa-gated Cl- channels + what they’re used to treat
Benzodiazepines (e.g diazepam) used to treat anxiety.
Barbiturates are sedatives + anti-convulsants.
Neurosteroids are metabolites of steroid hormones (e.g progesterone).
What happens due to constant inhibition? Using example of alcoholics
Constant increase in inhibition = cell decrease number of receptors to return to normal level on inhibition. Alcoholics stop drinking = withdrawal symptoms such as seizures as there is far less inhibition than there was previously.
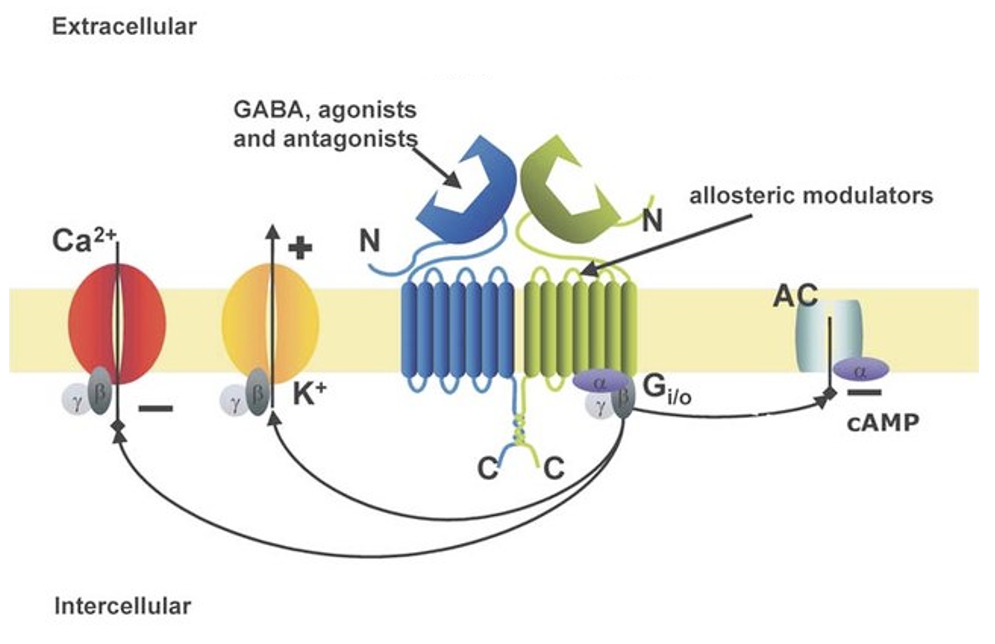
Describe metabotropic GABAb receptors
Are GPCR. Can in different ways in different cells but might: open K+ channels, close Ca2+ channels, trigger other second messengers like cAMP. Often presynaptic or auto-inhibitory (neuron released GABA to inhibit itself, type of feedback loop).
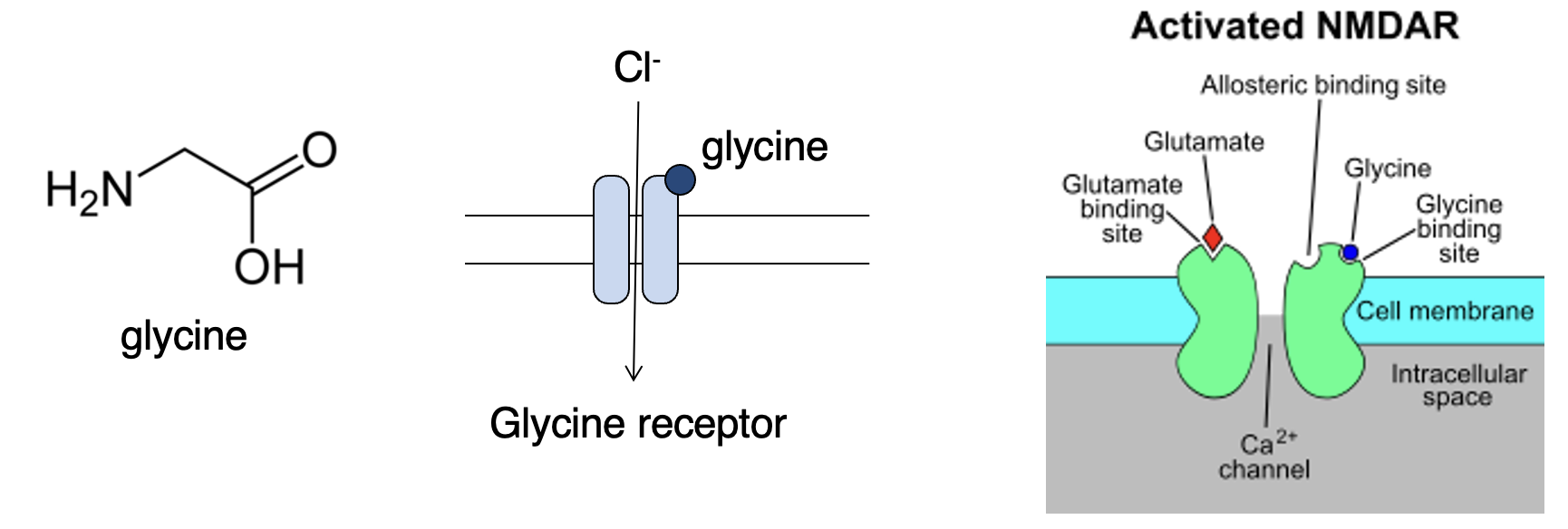
Describe glycine
Inhibits neurons via glycine-gated chloride channel (glycine receptor). Also binds to NMDA glutamate receptors. Can have diff effects depending on context.
Give an example of when GABAa receptors don’t always produce an IPSP
If membrane potential (Vm) is near chloride’s Nernst potential.
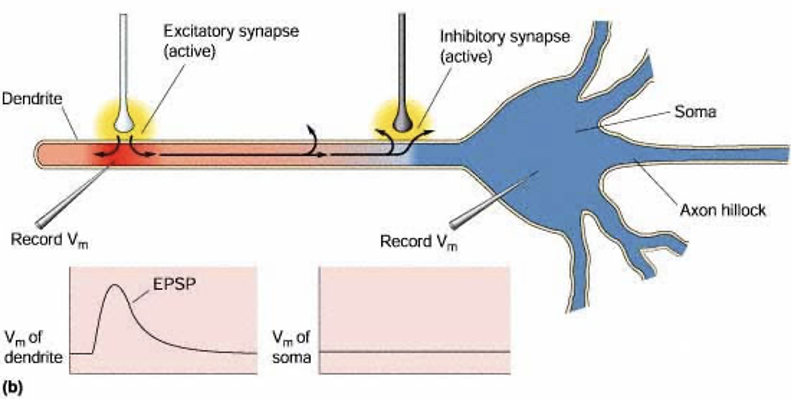
What can an inhibitory synapse block?
Propagation of an EPSP towards the soma.
What happens when chloride conductance is opened?
Membrane resistance decreases = current leaks out the membrane. D
Describe shunting inhibition
Excitation + inhibition = Cl- ions flow in (as reaches threshold that allows them to flow down electrochemical gradient) = get shunting inhibition (+ive current leaks out membrane at point where all Cl- is entering) = EPSP gets stopped = less likely to get transfer of depolarisation.
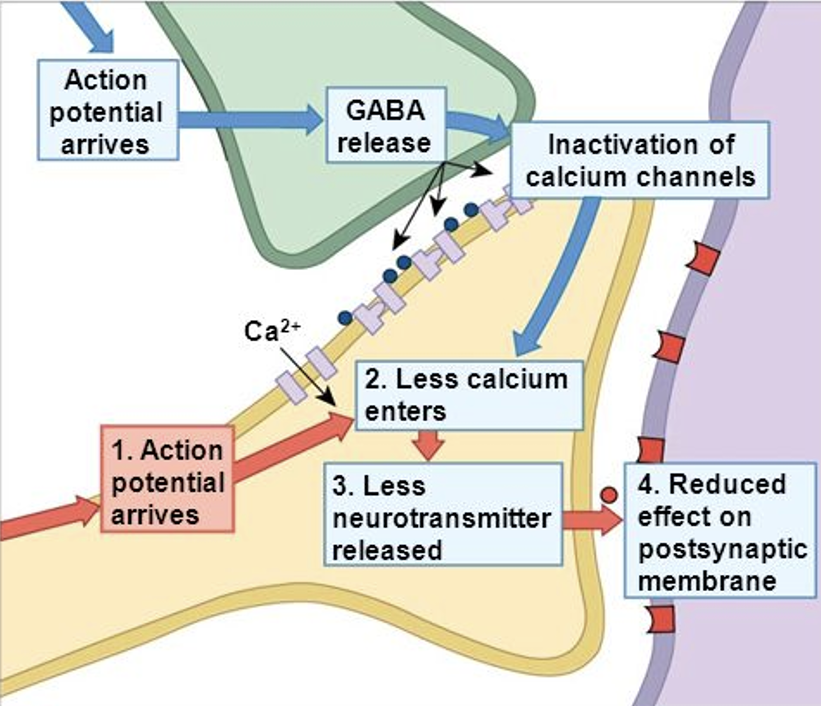
What happens if presynaptic GABA is present?
Presynaptic GABA = Ca2+ channels close = less Ca2+ entering cell + less NT released = reduced effect on postsynaptic membrane. Way of modulating NT released.
What do inhibitory neurons do?
Shape behaviour/activity of excitatory neurons. Often used as interneurons. Modules how much excitation in system. Need to balance inhibitory + excitatory.
What is acety CoA produced by?
Cellular respiration in mitochondria.
What does acetylcholinesterase do + where does it act?
Breaks down ACh in the synaptic cleft into acetic acid + choline.
What is combined to make acetylcholine?
Acetyl CoA + choline.
Why is choline taken back up by a choline transporter?
To be recycled to make more ACh.
What types of receptors does ACh act on?
Nicotinic (inotropic) + muscarinic (metabotropic).
Describe nicotinic receptors (nAChRs)
ACh-gated Na+/Ca2+ channel. Found at neuromuscular junction. CNS.
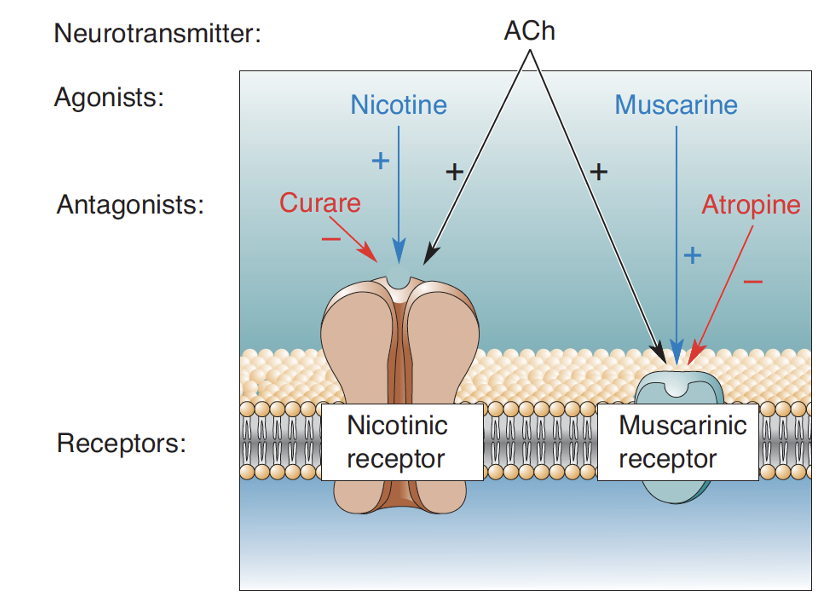
Describe muscarinic receptors (mAChRs)
5 types of GPCRs. Found in CNS + autonomic NS. More of these in brain compared to nicotinic receptors.
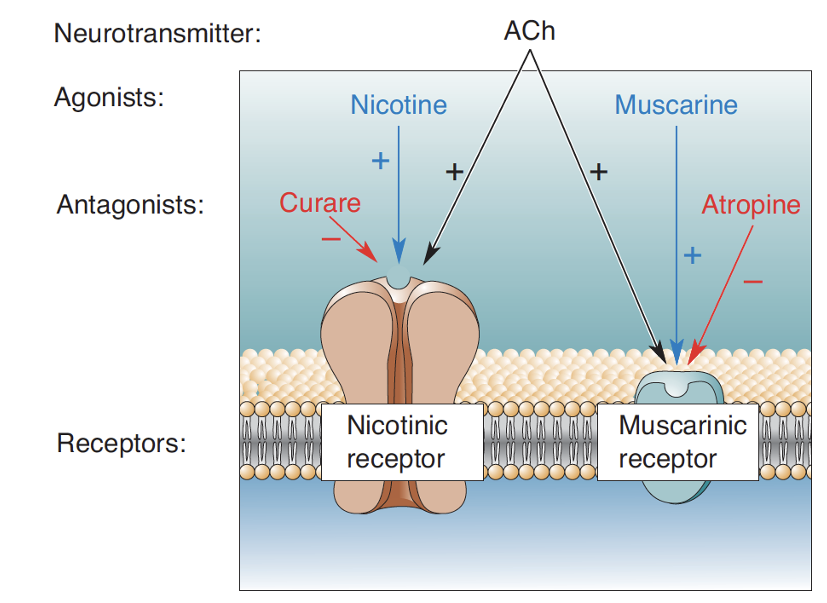
What type of receptors receive the signal on muscles at the NMJ? + why
Nicotinic ACh receptors. Because ACh is the NT at the NMJ.
What happens when ACh release is blocked? + give 2 examples of what can cause this
Block release from motor nerve = asphyxiation + lungs can’t move = death. Botulinum toxin + black widow spider venom.
What happens when acetylcholinesterase (AChE) is blocked? + give 2 examples of what can cause this
Block AChE = messes up breakdown of ACh = impacts signal of parasympathetic NS = ACh builds up = functioning of organs is damaged. Nerve gas, organophosphate pesticides, Alzheimer’s treatments.
What happens when ACh receptors are blocked? + give examples of what can cause this in nicotinic + muscarinic receptors
Paraylsis. Nicotinic: curare, alpha-bungarotoxin. Muscarinic: atropine.
Why is atropine used to treat nerve gas poisoning?
Binds + blocks muscarinic ACh receptors = prevents buildup of ACh from continuing to affect the receptor.
What are monoamines synthesised from?
a.a.
Describe the storage and removal of monoamines
Packed into vesicles by vesicular monoamine transporters (VMAT). Removed from synaptic cleft by re-uptake transporters (specific ones for each monoamine). Destroyed by monoamine oxidase (MAO) + catechol-O-methyltransferase (COMT, on posynaptic cell, only for catecholamines not serotonin).
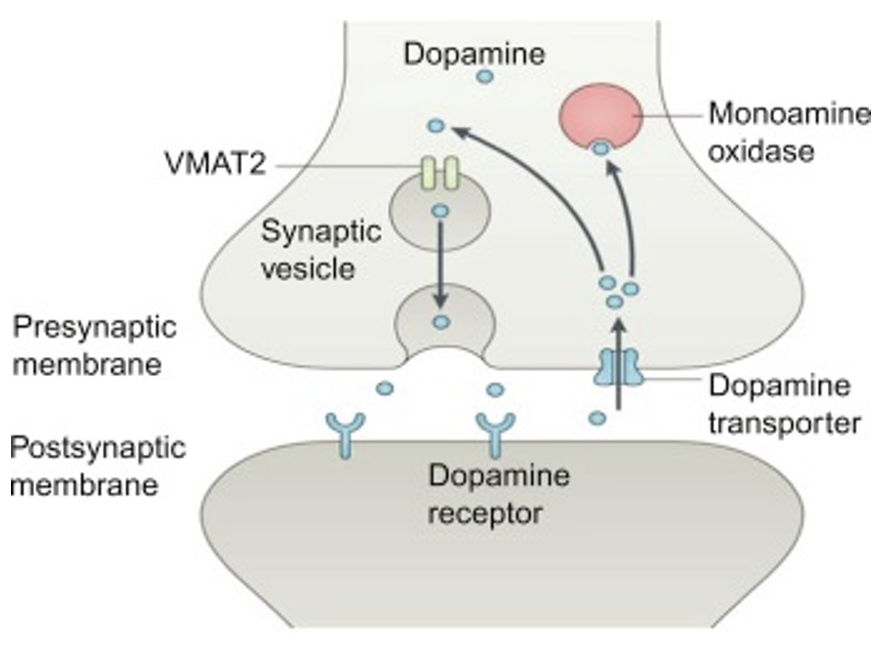
Why can drugs have specific effects?
Diff receptors are expressed in diff neuron types or parts of the brain.
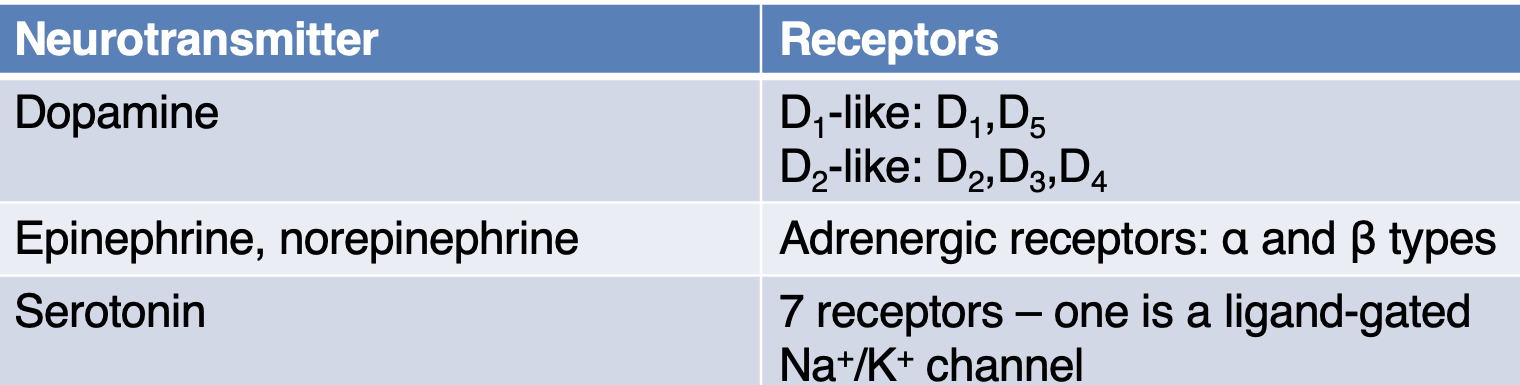
State the receptors the following NTs bind to: dopamine, epinephrine, norepinephrine, serotonin
State 2 functions of dopamine
Motor control + reward.
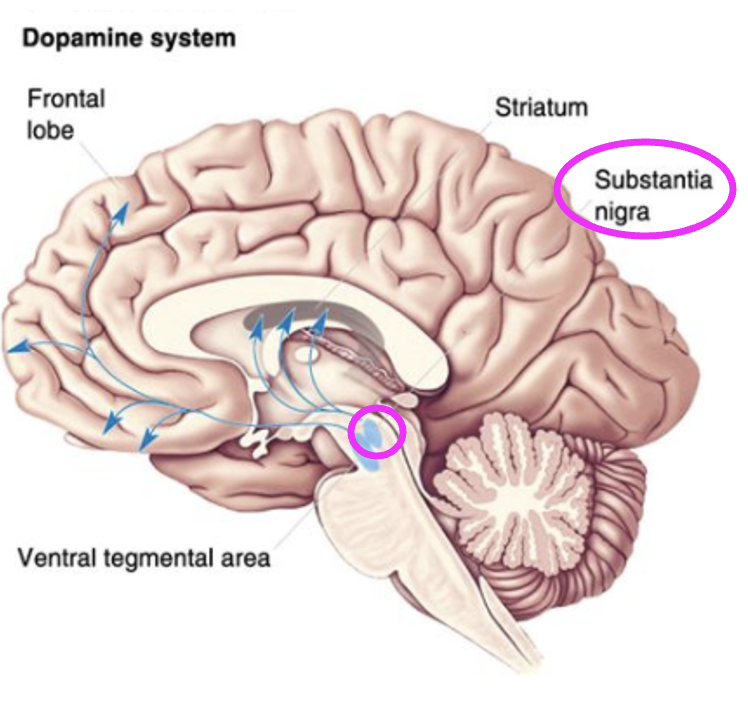
What does the nigrostriatal pathway do?
Facilitates initiation of voluntary movement.
What neurons in the substantial nigra project to the striatum? + in what disease do these neurons die in?
Dopaminergic neurons. Parkinson’s disease.
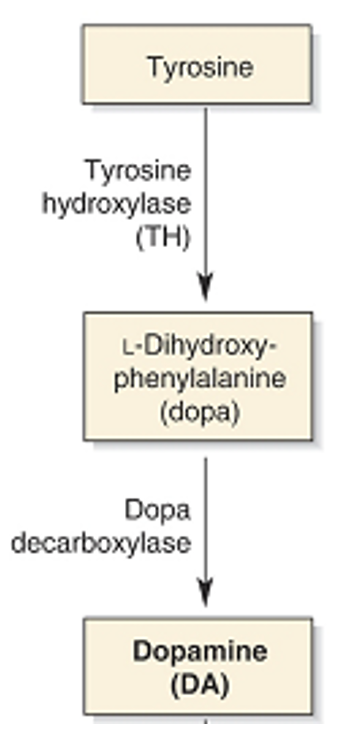
What is dopamine synthesised from?
L-DOPA which is synthesised from tyrosine.
Why can’t you give dopamine to a Parkinson’s patient? + what can be given instead?
Dopamine can’t cross the blood brain barrier so it would be ineffective. Can give L-DOPA instead.
What can be given in the early stages of Parkinson’s disease when all the dopamine receptors haven’t died yet to enhance the functions of the receptors remaining?
MAO-B inhibitors = increase dopamine = increase function of neurons in the nigrostriatal pathway.
What does the mesolimbic pathway mediate?
Reward/motivation.
Dopaminergic neurons in which area project to the cortex and limbic system?
Ventral tegmental area (VTA).
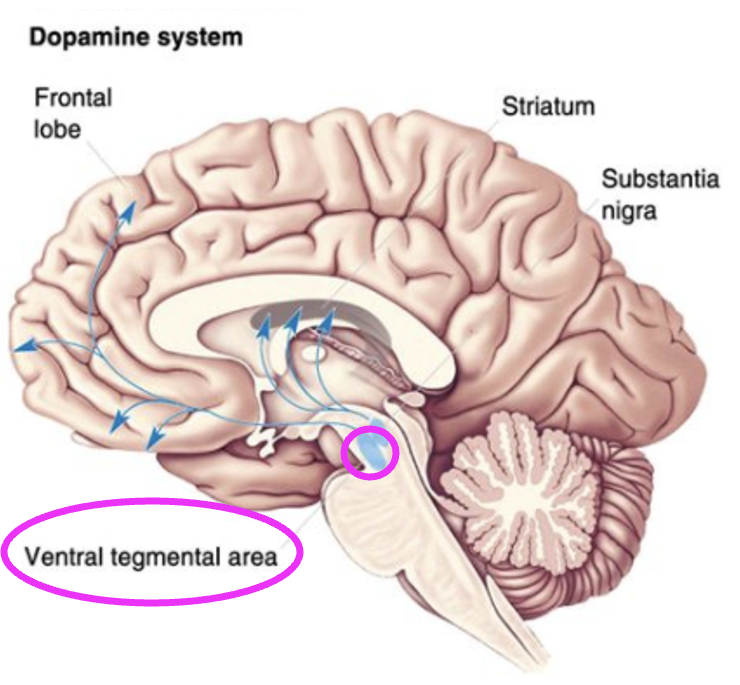
Which brain circuit is a likely target for addiction?
Mesolimbic.
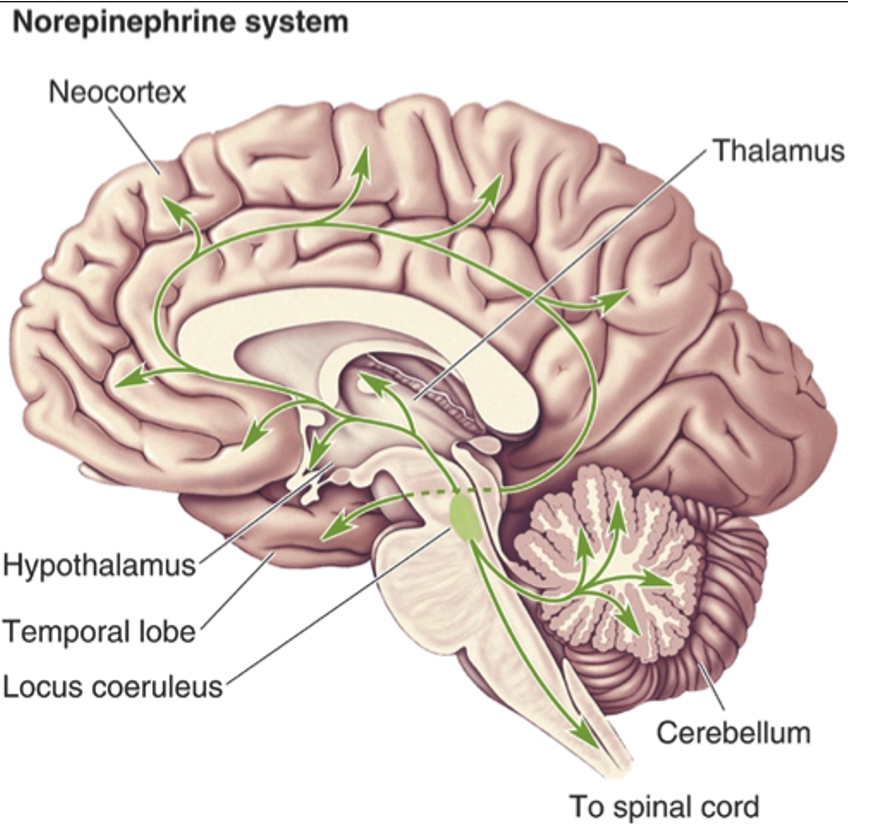
What do noradrenergic neurons regulate?
Arousal, attention, mood, memory, anxiety, pain.
What type of neurons are found in the locus coeruleus which innervate the whole brain?
Noradrenergic neurons.
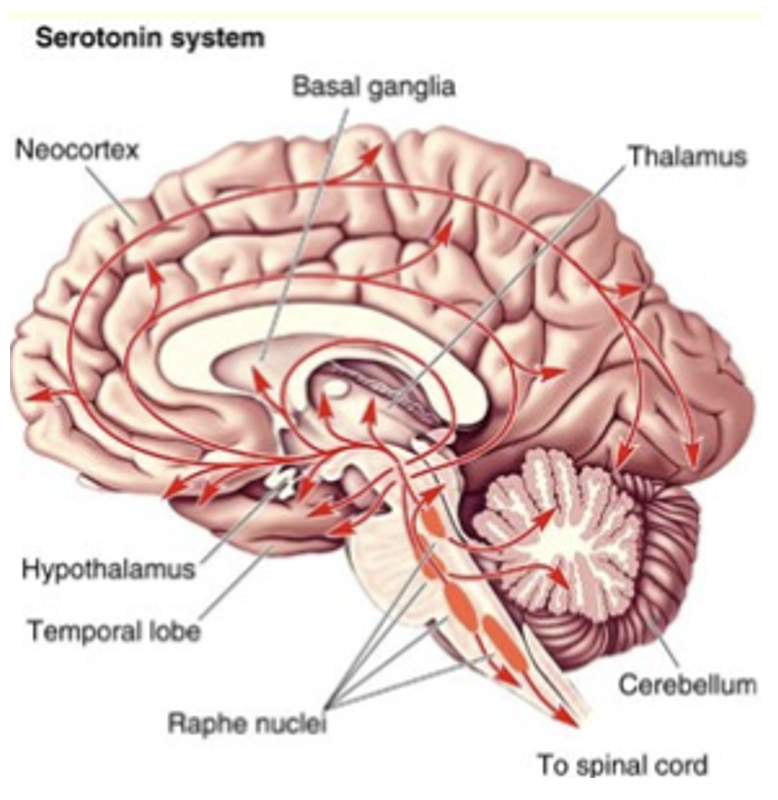
What do serotonergic neurons regulate?
Sleep/wake, mood.
Which neurons are found in the Raphe nuclei + project all over the brain?
Serotonergic.
Describe the effect of cocaine + amphetamines
Block dopamine reuptake transporter = dopamine stays in synaptic cleft longer = enhances dopamine signalling = very rewarding. Overdose = increase HR to fatal level.
What do antipsychotics block?
Dopamine receptors.
What do tricyclics do?
Block transporters for serotonin + norepinephrine. Is antidepressant.
What do MAO-A inhibitors do?
Stop serotonin from being destroyed. Is antidepressant.
What do opioid peptides (endorphins) do?
NT. Bind to opioid receptors (GPCRs). Regulate pain (perception/emotion/cognitive). Regulate coughing, GI tract. Receptors are targets of morphine, heroin.